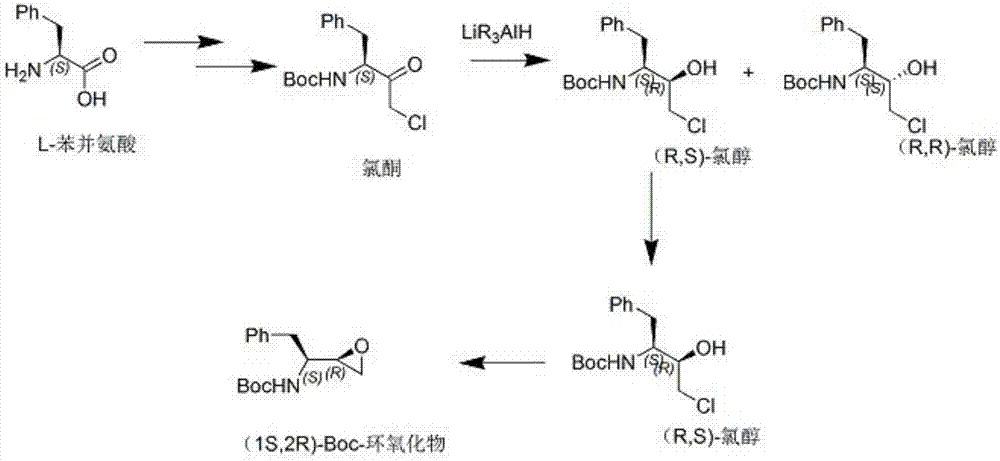
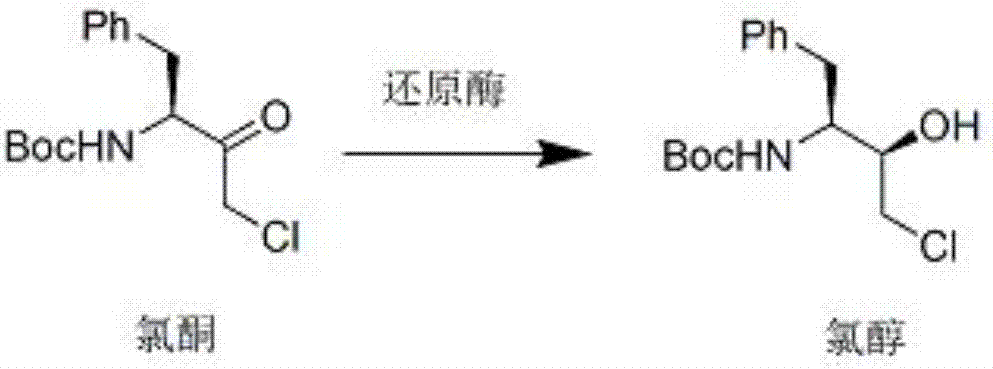
Method used for synthesis of atazanavir intermediate chlorhydrin via bio-enzyme catalysis
A technology of atazanavir and chlorohydrin, which is applied in the field of preparation of atazanavir intermediate chlorohydrin selectively catalyzed by biological enzymes, can solve the problem of low selectivity, complex fermentation process of Rhodococcus erythroflates, and difficulty in industrialization, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 300g of methyl tertiary ether, 0.5g of reductase KRED-101, and 35g of purified water into a 1L four-necked reaction flask. After adding, keep the temperature at 25-30°C for 30 minutes, then cool down to 10-15°C and add chlorine in batches. Ketone 29g, control the temperature between 10-15°C, add about 1.5 hours, maintain at 10-15°C for 24 hours, control in the middle, after the reaction is completed, add 300g of purified water at 0°C, and then add 300g of purified water at 0°C Add 50g of 18% hydrochloric acid, stir for 0.5 hours, naturally warm up to room temperature, then separate the upper layer of tertiary methyl ether, and extract the aqueous layer with methyl tertiary ether (2×80g), combine the organic layers, wash with 100g of saturated sodium chloride solution, 40g of anhydrous sodium sulfate was dried and filtered, and the filtrate was concentrated, then added 100g of n-hexane and heated to reflux for dissolution, then cooled to 15-20°C, crystals were precipi...
Embodiment 2
[0025] When other conditions are consistent with Example 1, the reductase is replaced by KRED-129, and the ratio of (1S,2R)-BOC-epoxide to (1R,2R)-BOC-epoxide obtained by reducing ketone is 35:65 .
Embodiment 3
[0027] When other conditions are consistent with Example 1, the reductase is replaced by KRED-131, and the ratio of (1S,2R)-BOC-epoxide to (1R,2R)-BOC-epoxide obtained by reducing ketone is: 59: 31.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com