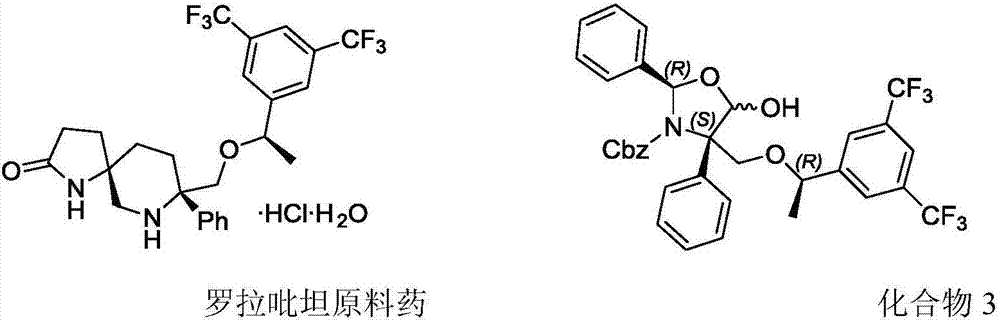
Method for preparing medical intermediate for tumor chemotherapy
An intermediate and pharmaceutical technology, applied in the field of preparation of pharmaceutical intermediates, can solve the problems of unsuitability for large-scale industrial production, low yield of target products, and low yield, and achieve high chiral purity of products and simplified post-processing methods , the effect of high reaction purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
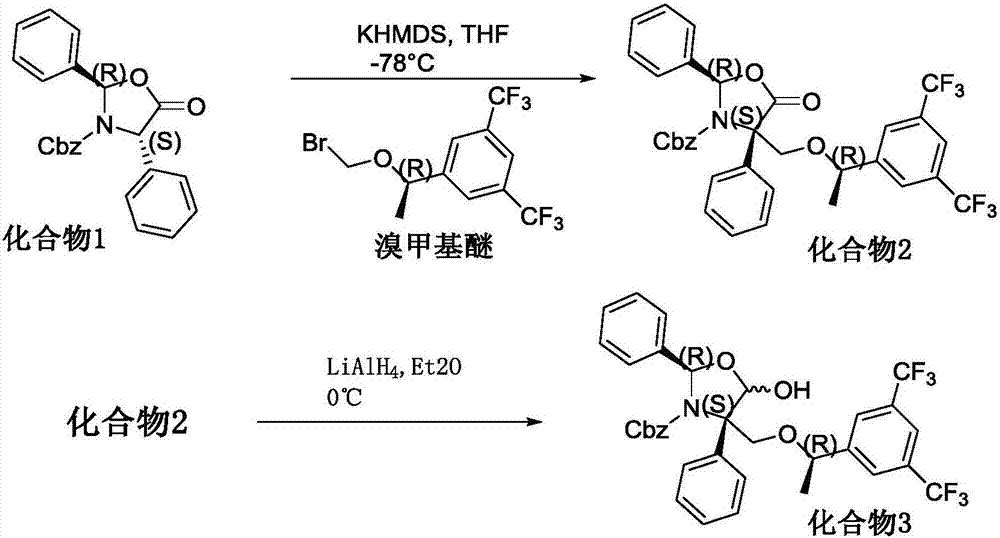
[0032] The present invention is used for the preparation method of the pharmaceutical intermediate of preventing and treating tumor chemotherapy, comprises the following steps:
[0033] S1. Preparation of compound 2 using compound 1:
[0034] Include the following sub-steps:
[0035] a. Add compound 1 in tetrahydrofuran solvent, cool to -60~85°C, add lithium hexamethyldisilazide, stir and mix evenly, add bromomethyl ether to obtain a mixed solution, and stir the mixed solution for 1-2 hours Add saturated ammonium chloride solution to quench the reaction, use ethyl acetate to extract and separate the layers to obtain the organic layer, wash the organic layer with saturated sodium chloride, add anhydrous sodium sulfate to dry the organic layer, filter the dried organic layer to obtain the filtrate, After concentrating the dry filtrate under reduced pressure, adding petroleum ether, cooling and crystallizing, filtering, and washing the filter cake with petroleum ether to obtai...
Embodiment 2
[0043] Based on Example 1, this example is a specific implementation method: add anhydrous tetrahydrofuran (465L) to a 1000L stainless steel reactor, the moisture content of tetrahydrofuran is 0.02%, add compound 1 (15.50kg, 415mol) and stir. Under nitrogen protection, cool to -70±3°C. At -70±3°C, lithium hexamethyldisilazide tetrahydrofuran solution (49.8 L, concentration 1 mol / L) was added and stirred for 1 hour. A tetrahydrofuran solution (50% strength) of bromomethyl ether (17.49 kg, 498 mol) was added at -60°C to 70°C. After the addition, naturally raise the temperature and stir for 2 hours, add 200kg of saturated ammonium chloride solution to quench, extract with 140kg of ethyl acetate, separate liquid at 12±6°C, wash with 285kg of saturated sodium chloride, add 60kg of anhydrous sodium sulfate to dry the organic layer for 3 hours , filter, and concentrate the filtrate under reduced pressure at 45°C. After concentrating to dryness, add 50.38kg of petroleum ether and cool ...
Embodiment 3
[0046] Based on Example 1, add anhydrous tetrahydrofuran (3000ml) into the reaction kettle, the moisture content of tetrahydrofuran is ≤0.03%, add 100g of compound 1 and stir, under nitrogen protection, cool to -73°C in the freezing liquid, add hexamethyldisilazylamino Lithium tetrahydrofuran solution (321.4ml, concentration 1mol / L), stirred for 2 hours, added bromomethyl ether tetrahydrofuran solution (50% concentration, containing bromomethyl ether 112.83g) at -70°C, removed the cooling liquid bath after adding Stir for 3 hours at natural temperature, add 1000ml of saturated ammonium chloride solution to quench, extract with 1000ml of ethyl acetate, and separate liquids at 12±6°C. Wash with 1300ml of saturated sodium chloride, add 400g of anhydrous sodium sulfate to dry the organic layer for 2 hours, filter, and concentrate the filtrate under reduced pressure at 45°C. , washed the filter cake with petroleum ether to obtain the wet product of Compound 2; the filtrate lotion w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com