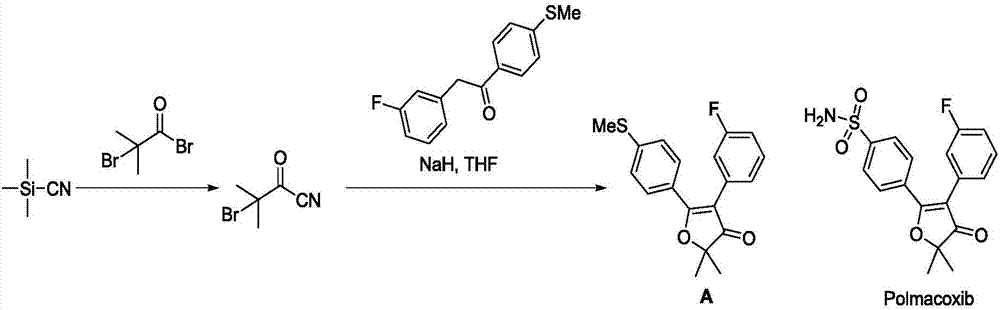
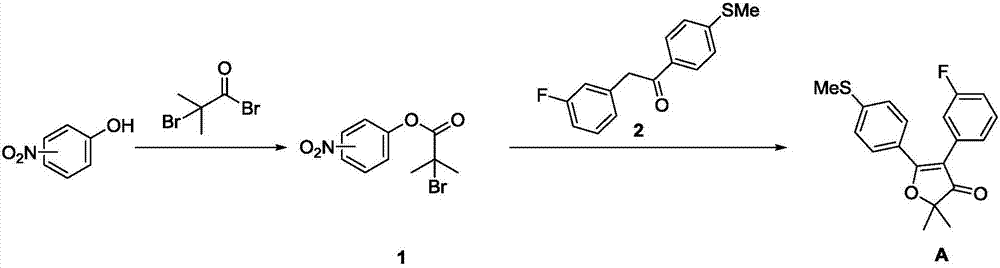
Synthesis method of non-steroidal anti-inflammatory drug, polmacoxib key intermediate
A non-steroidal anti-inflammatory drug, pomacoxib technology, applied in the field of synthesis of key intermediates of non-steroidal anti-inflammatory drug pomacoxib, can solve the problem of difficult treatment of waste water and waste residue, and achieve less environmental pollution and easy The effect of low processing and equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Step 1: Synthesis of 2-bromo-2-methyl-propionic acid-(4-nitro)phenol ester (compound 1)
[0029] Dissolve 30.1g of 4-nitrophenol in 300mL of dichloromethane, add 24.1g of triethylamine dropwise at 0-10°C, and then add 50.2g of bromoisobutyryl bromide dropwise. After the dropwise addition, stir at room temperature for 0.5 hour, then add water to quench the reaction, separate the organic phase, wash the organic phase with 0.5M dilute acid and 5% sodium carbonate solution successively, dry over anhydrous sodium sulfate, filter, concentrate, obtain compound 1 after beating with petroleum ether, and the yield 95.6%.
[0030] Step 2: Synthesis of 2,2-dimethyl-4-(3-fluorophenyl)-5-[4-(methylthio)phenyl]-3(2H)furanone (compound A);
[0031] Dissolve 26.5g of compound 1 and 10.4g of compound 2 in tetrahydrofuran, cool down to -20°C, slowly add sodium hexamethyldisilazide (2M, 57.2mL) dropwise, after the dropwise addition, stir for half an hour, add water to quench The reaction...
Embodiment 2
[0033] Step 1: Synthesis of 2-bromo-2-methyl-propionic acid-(2-nitro)phenol ester (compound 1)
[0034] Dissolve 30.1g of 2-nitrophenol in 300mL of dichloromethane, add 30.8g of diisopropylethylamine dropwise at 0-10°C, then add 50.2g of bromoisobutyryl bromide dropwise, after the dropwise addition , stirred at room temperature for 0.5 hours, then added water to quench the reaction, separated the organic phase, and washed the organic phase with 0.5M dilute hydrochloric acid and 5% sodium carbonate solution successively, dried over anhydrous sodium sulfate, filtered, concentrated, and obtained the compound after beating with n-heptane 1. The yield is 91.8%.
[0035] Step 2: Synthesis of 2,2-dimethyl-4-(3-fluorophenyl)-5-[4-(methylthio)phenyl]-3(2H)furanone (compound A);
[0036] Dissolve 26.5g of compound 1 and 10.4g of compound 2 in tetrahydrofuran, cool down to -20°C, slowly add sodium hexamethyldisilazide (2M, 57.2mL) dropwise, after the dropwise addition, stir for half an ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com