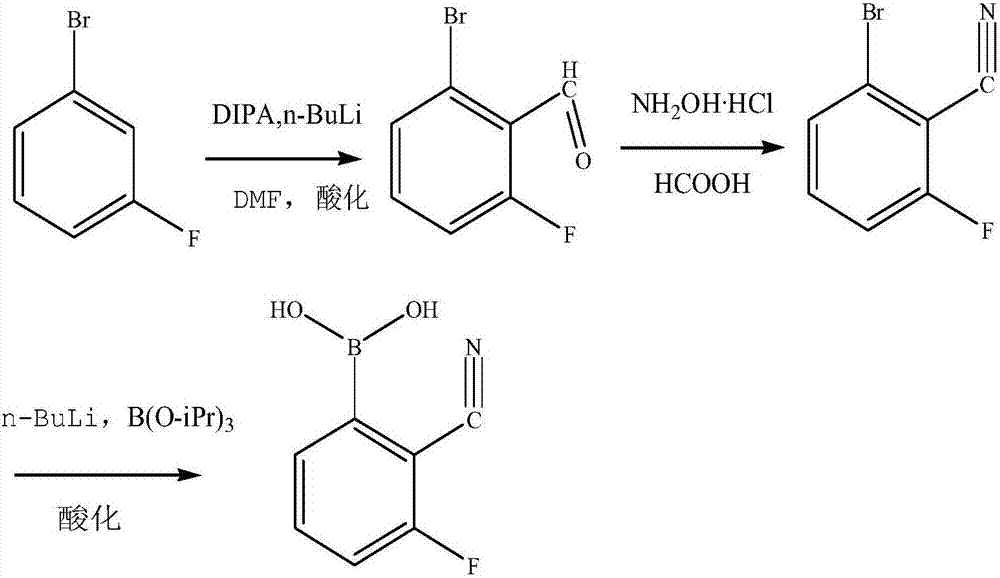
Process for synthesizing 2-cyanogroup 3-fluorophenylboronic acid
A technology of fluorophenylboronic acid and cyano group is applied in the field of synthesis of 2-cyano 3-fluorobenzeneboronic acid, which can solve the problems of no relevant literature reports, and achieve the effects of improving product yield, good process stability and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] a) Synthesis of 2‐fluoro6‐bromobenzaldehyde: Add 0.66mol of diisopropylamine and 50g of anhydrous ether to a nitrogen-protected four-necked flask, drop the temperature to -40°C to -50°C, add 1.25mol of n-butyllithium dropwise, and stir Keep warm for 1 hour, and the reaction of raw materials is completed. Cool down to -70°C to -80°C, add dropwise a mixed solution of 0.29mol m-fluorobromobenzene and 120g anhydrous ether, and stir for 2 hours after the dropwise addition. Afterwards, 0.38 mol of dimethylformamide was added dropwise, stirred for 1 hour, and quenched with acetic acid at pH=1-2. The oil layer was extracted with methyl tert-butyl ether and distilled under vacuum under reduced pressure to obtain the intermediate 2-fluoro-6-bromobenzaldehyde with a GC purity of 99%.
[0028] b) Synthesis of 2-fluoro-6-bromoxynil: Add 0.2mol of 2-fluoro-6-bromobenzaldehyde, 0.4mol of hydroxylamine hydrochloride, and 150g of formic acid into a 1L single-necked bottle, heat up at 1...
Embodiment 2
[0031] a) Synthesis of 2‐fluoro6‐bromobenzaldehyde: Add 268 g of diisopropylamine and 100 g of tetrahydrofuran into a nitrogen-protected four-neck flask, cool down to -40°C to -50°C, and dropwise add 340 g of Butyllithium was stirred and kept warm for 1 hour, and the reaction of the raw materials was completed. Cool down to -70°C to -80°C, add dropwise a mixed solution of 200g m-fluorobromobenzene and 100g tetrahydrofuran, and stir for 2 hours after the dropwise addition. Afterwards, 112 g of dimethylformamide was added dropwise, stirred for 1 hour, and quenched with hydrochloric acid to pH=1-2. Ethyl acetate extracted the oil layer and distilled it under vacuum under reduced pressure to obtain 165 g of 2-fluoro-6-bromobenzaldehyde with a GC purity of 98%.
[0032] b) Synthesis of 2-fluoro-6-bromoxynil: add 165g of 2-fluoro-6-bromobenzaldehyde, 114g of hydroxylamine hydrochloride, and 450g of formic acid into a 2L single-necked bottle, heat up at 100°C and reflux for 2 hours,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com