Method of preparing methyl ketone through cobalt catalysis
A technology for catalytic preparation and methyl ketone, applied in the field of chemistry, can solve the problems of incapable of large-scale production, harsh reaction conditions, expensive catalysts, etc., and achieve the effects of fast reaction speed, mild reaction temperature, safety and feasibility guarantee.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
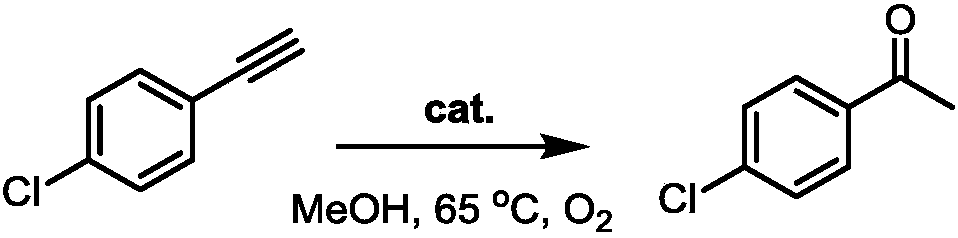
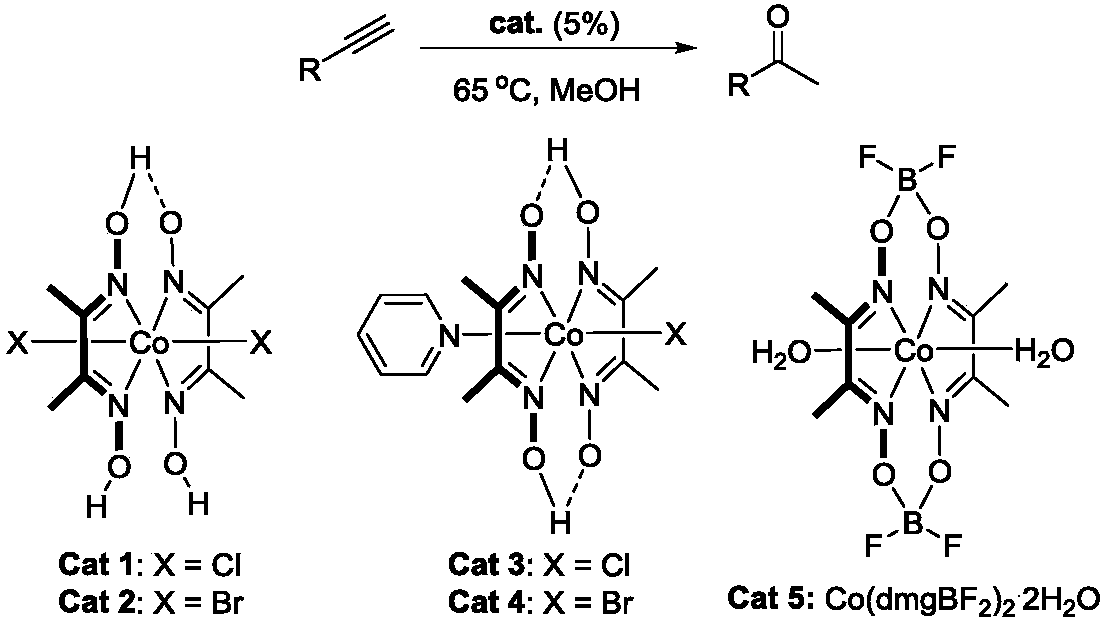
[0009] P-Chlorophenylacetylene (10g, 73.2mmol) was added to a 250mL round bottom flask filled with methanol (117mL) solution, a magnet was added, and after stirring evenly, the catalyst (616mg, 1.46mmol) was added thereto. Then the reaction system was heated to 65° C., and after 20 h of reaction, it was quenched with water, and the reaction bottle was cooled to room temperature. After spinning off the solvent, it was separated by silica gel column chromatography to obtain a colorless oily liquid.
[0010] The reaction formula is:
[0011]
[0012] The following examples are helpful in understanding the present invention. In the table below are examples of the hydrolysis of alkynes to methyl ketones. Reaction conditions refer to embodiment example 1.
[0013]
[0014]
[0015]
[0016]
[0017]
Embodiment 2
[0019] Add 0.25mmol of an alkynyl compound (such as phenylacetylene, p-methoxyphenylacetylene, etc.) linked to an aromatic system into a 5mL round-bottomed flask filled with 1mL of methanol solution, add a magnet, and after stirring evenly, add 5mol% catalyst in. Then the reaction system was heated to 65° C., and after 4.5 h of reaction, it was quenched with water, and the reaction bottle was cooled to room temperature. After spinning off the solvent, it was separated by silica gel column chromatography to obtain a colorless oily liquid.
Embodiment 3
[0021] Add 0.25mmol of an acid-sensitive terminal alkyne compound (such as a compound containing Boc-, MOMO-, etc.) into a 10ml sealed tube containing 1mL of methanol solution, add a magnet, and after stirring evenly, add 5mol% catalyst in. Then the reaction system was heated to 60° C., and after 10-20 h of reaction, it was quenched with water, and the reaction bottle was cooled to room temperature. After spinning off the solvent, it was separated by silica gel column chromatography to obtain a colorless oily liquid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com