A heavy metal ion remover with self-flocculation function and its synthesis method
A technology of heavy metal ions and self-flocculation, which is applied in chemical instruments and methods, flocculation/sedimentation water/sewage treatment, water/sludge/sewage treatment, etc., can solve the problem of increased treatment cost and difficulty of treatment process, high heavy metal content, inability to Achieve the effect of good self-flocculation function, large molecular weight and good applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
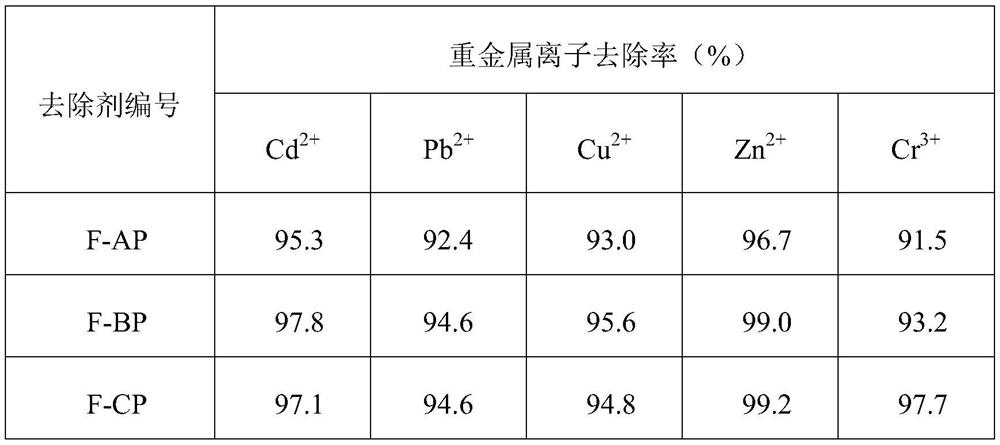
Embodiment 1
[0027] Step (1): Add 180g of sodium hydroxide aqueous solution (mass concentration: 3wt.%) to a three-necked flask with magnetic stirring, heat up to 45°C, then add 3g of soluble starch, after stirring evenly, slowly add 25g of epoxy Chloropropane, the system was slowly raised to 55°C and stirred for 8 hours. After the reaction was completed, it was cooled to room temperature, filtered, washed with distilled water, ethanol, and acetone in sequence, and vacuum-dried to obtain the target product AP1.
[0028] Step (2): Add 90g of sodium hydroxide aqueous solution (mass concentration is 0.5wt.%) to the three-necked flask with magnetic stirring, heat up to 60°C, then add 3g of the product AP1 synthesized in step (1), and stir evenly , slowly drop 9g of diethylenetriamine, and the system was stirred and reacted at 60-70°C for 4 hours. After the reaction was completed, it was cooled to room temperature, filtered, washed with distilled water, ethanol, and acetone in sequence, and va...
Embodiment 2
[0033] Step (1): Add 200g of sodium hydroxide aqueous solution (mass concentration: 2wt.%) to a three-necked flask with magnetic stirring, heat up to 55°C, then add 3g of soluble starch, after stirring evenly, slowly add 30g of epoxy Chloropropane, the system was slowly raised to 60°C and stirred for 6 hours. After the reaction was completed, it was cooled to room temperature, filtered, washed with distilled water, ethanol, and acetone in turn, and vacuum-dried to obtain the stage target product BP1.
[0034] Step (2): Add 120g of sodium hydroxide aqueous solution (mass concentration is 1.5wt.%) to the there-necked flask with magnetic stirring, heat up to 65°C, then add 3g of the product BP1 synthesized in step (1), and stir evenly 12 g of triethylenetetramine was slowly added dropwise, and the system was stirred and reacted at 70° C. for 6 hours. After the reaction was completed, it was cooled to room temperature, filtered, washed with distilled water, ethanol, and acetone i...
Embodiment 3
[0039] Step (1): Add 240g of sodium hydroxide aqueous solution (mass concentration is 4wt.%) to the three-necked flask with magnetic stirring, heat up to 55°C, then add 3g of soluble starch, after stirring evenly, slowly add 40g of epoxy Chloropropane, the system was slowly raised to 70°C and stirred for 12 hours. After the reaction was completed, it was cooled to room temperature, filtered, washed with distilled water, ethanol, and acetone in sequence, and vacuum-dried to obtain the stage target product CP1.
[0040] Step (2): Add 150g potassium hydroxide aqueous solution (mass concentration is 2wt.%) to the there-necked flask with magnetic stirring, heat up to 65°C, then add 3g of the product CP1 synthesized in step (1), after stirring evenly, 15 g of tetraethylenepentamine was slowly added dropwise, and the system was stirred and reacted at 75-80° C. for 8 hours. After the reaction was completed, it was cooled to room temperature, filtered, washed with distilled water, eth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com