A kind of gefitinib tablet and preparation method thereof
A technology of gefitinib and nigra tablets, applied in the field of pharmaceutical preparations, can solve the problems of non-compliance with green chemistry, damage to instruments and equipment, lactose intolerance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
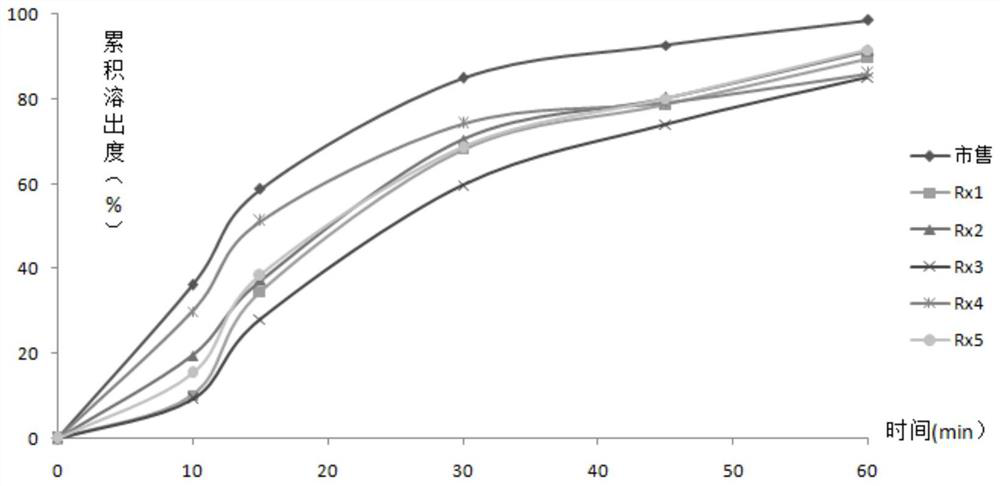
[0063] In order to avoid lactose intolerance, pregelatinized starch and microcrystalline cellulose 101 were used as fillers, and the traditional wet granulation process was used to compress tablets. The basic prescription was designed as shown in Table 1. By selecting different disintegrants, Croscarmellose sodium, carboxymethyl starch sodium, carboxymethylcellulose sodium, crospovidone XL-10 and low-substituted hydroxypropyl cellulose were used to investigate the disintegration and dissolution of gefitinib tablets .
[0064] Table 1 Selection of disintegrants
[0065]
[0066]
[0067] According to "Chinese Pharmacopoeia" 2015 edition four general rules 0921 to determine the disintegration time limit. It can be seen from Table 1 that Rx4 uses crospovidone XL-10 as the disintegrant, which has the fastest disintegration and can be disintegrated into large particles, while Rx1~Rx3 and Rx5 use croscarmellose sodium , sodium carboxymethyl starch, sodium carboxymethylcellul...
Embodiment 2
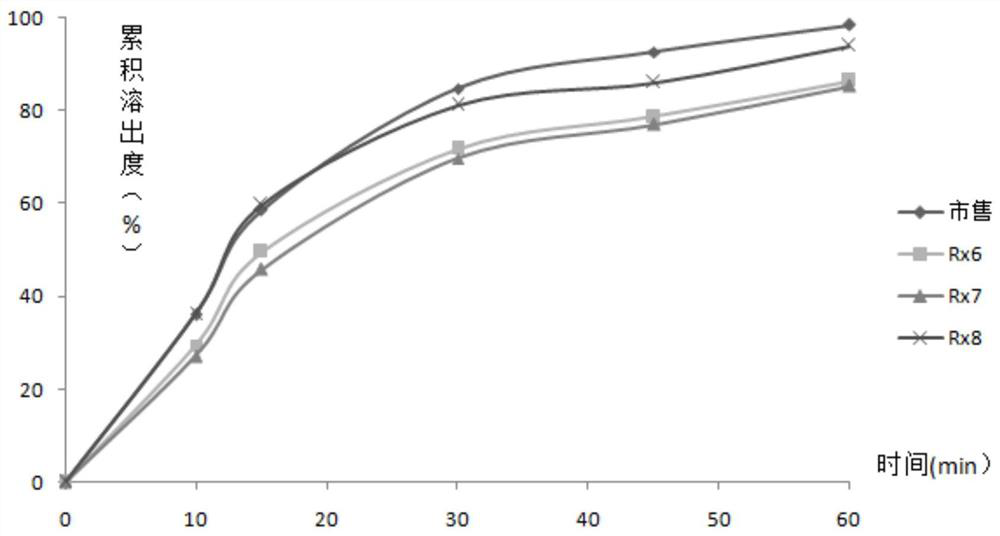
[0073] On the basis of Rx4, different granulation methods were selected to prepare gefitinib tablets, and the suitable process was determined by tablet disintegration and dissolution.
[0074] Preparation Process:
[0075] Pretreatment: Gefitinib micronization treatment, D50<3μM, D90<5μM.
[0076] a. Fluidized bed: Weigh the raw and auxiliary materials of the prescription amount respectively, add the materials into the fluidized bed, set the inlet air temperature to 55°C, the atomization pressure to 1.5MPa, and the air inlet volume to 30m 3 / h, granulate with appropriate parameters, spray the binder with the top spray method for granulation, dry at the same time, granulate with 20 mesh, add external materials for total mixing, and press into 11mm shallow concave tablets.
[0077] b. Powder direct compression: Weigh the raw and auxiliary materials of the prescription amount, mix them in equal increments, and press them into tablets with a shallow concave of 11 mm.
[0078] c....
Embodiment 3
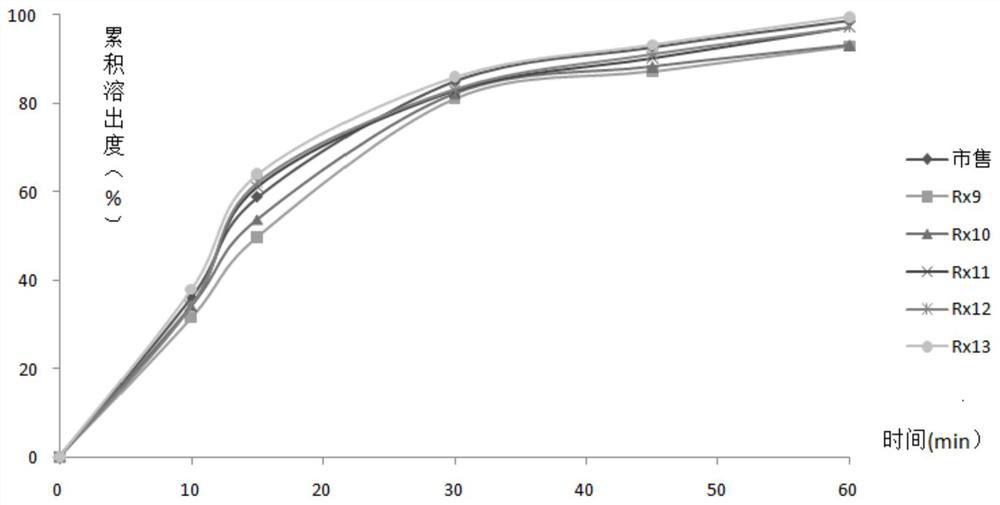
[0087] On the basis of Rx8, gefitinib tablets were obtained by dry granulation by adjusting the ratio of pregelatinized starch and microcrystalline cellulose, and the disintegration and dissolution of plain tablets were investigated. The results are shown in Tables 5 to 6 and attached image 3 .
[0088] Table 5 Investigation results of fillers with different proportions
[0089]
[0090]
[0091] It can be seen from Table 5 that the weight ratios of filler pregelatinized starch / microcrystalline cellulose in Rx9~Rx13 are 8:13, 10:11, 4:3, 2:1 and 16:5, respectively. The higher the ratio of pregelatinized starch / microcrystalline cellulose 101, the faster the disintegration of gefitinib tablets. It may be due to the high viscosity of microcrystalline cellulose, which affects the disintegration speed of plain tablets, so the amount of microcrystalline cellulose in the filler should be controlled. When the weight ratio of pregelatinized starch / micronised cellulose 101 is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com