Pharmaceutical composition for prevention or treatment of fibrotic diseases and application thereof
A technology of fibrotic diseases and compositions, applied in the field of medicine, can solve problems such as organ function decline and organ fibrosis, and achieve good prevention and treatment effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1: the preparation of nintedanib capsule
[0062] Nintedanib capsules were prepared according to the following method and the proportioning of materials in Table 1:
[0063] Table 1: Ratio of nintedanib capsules
[0064]
[0065] Heat the prescribed amount of medium-chain triglycerides, mixed fatty acid glycerides, and soybean lecithin to 40-50°C to melt, add the prescribed amount of nintedanib ethanesulfonate that has passed through a 0.8mm sieve, mix well, and press the In a quality machine (rotating speed 20000r / min), make 3-5 times, each time for 2 minutes, until the contents are in a completely uniform state, and the capsule liquid is obtained, which is filled into the capsule shell by pressing or dripping method, and is prepared into a soft capsule, that is, have to.
Embodiment 2
[0066] Embodiment 2: the physicochemical property of nintedanib capsule
[0067] According to two kinds of prescriptions of embodiment 1, prepare 200 soft capsules respectively, observe the appearance of soft capsules, check the difference in loading (the difference in loading must not exceed 7.5%), measure the dissolution rate (paddle method (two appendix XC of Chinese Pharmacopoeia version in 2015) The second method)), determination of content (respectively determine 0 days and accelerated January 40 ℃, RH75% content).
[0068] Liquid phase detection method: use octadecylsilane bonded silica gel as filler; use 0.1% trifluoroacetic acid as mobile phase A, acetonitrile as mobile phase B, perform gradient elution according to Table 2, and the flow rate is 1.0 mL per minute; The detection wavelength is 387nm, and the column temperature is 35°C.
[0069] Table 2: Gradient ratio composition of liquid phase eluent
[0070]
[0071] Content determination method: take 20 capsule...
Embodiment 3
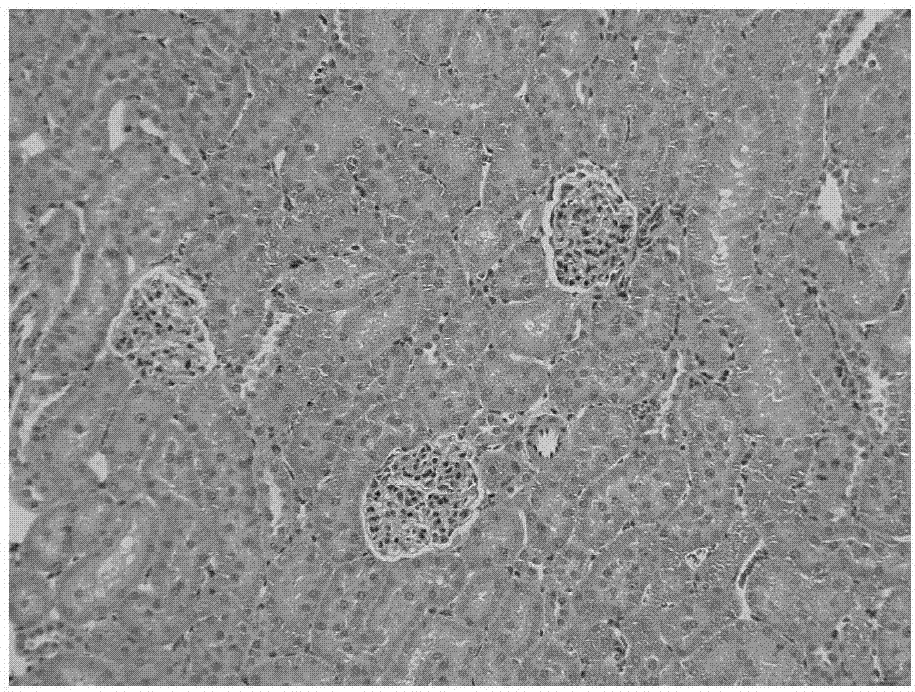
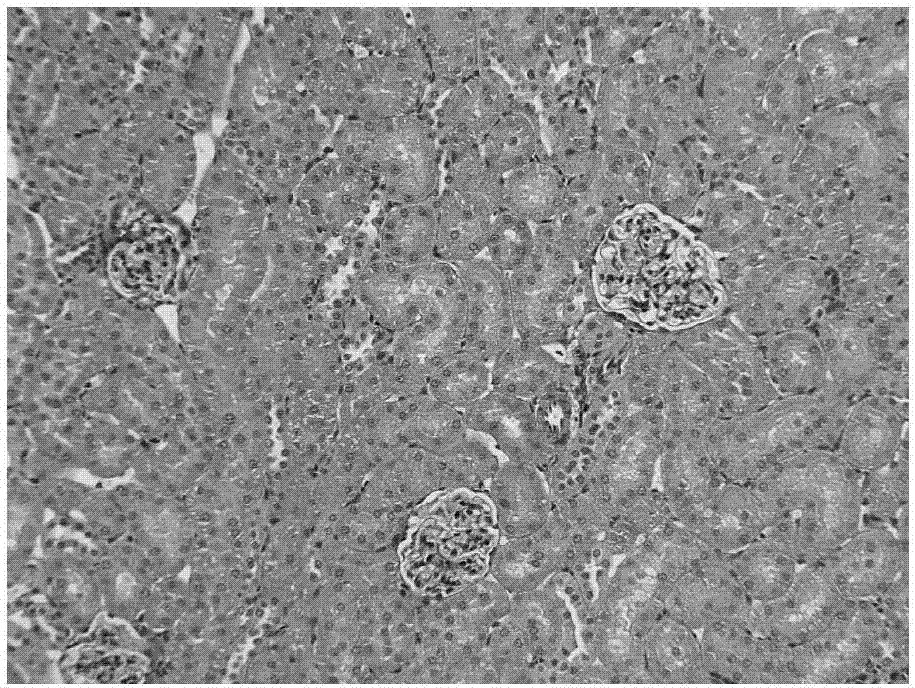
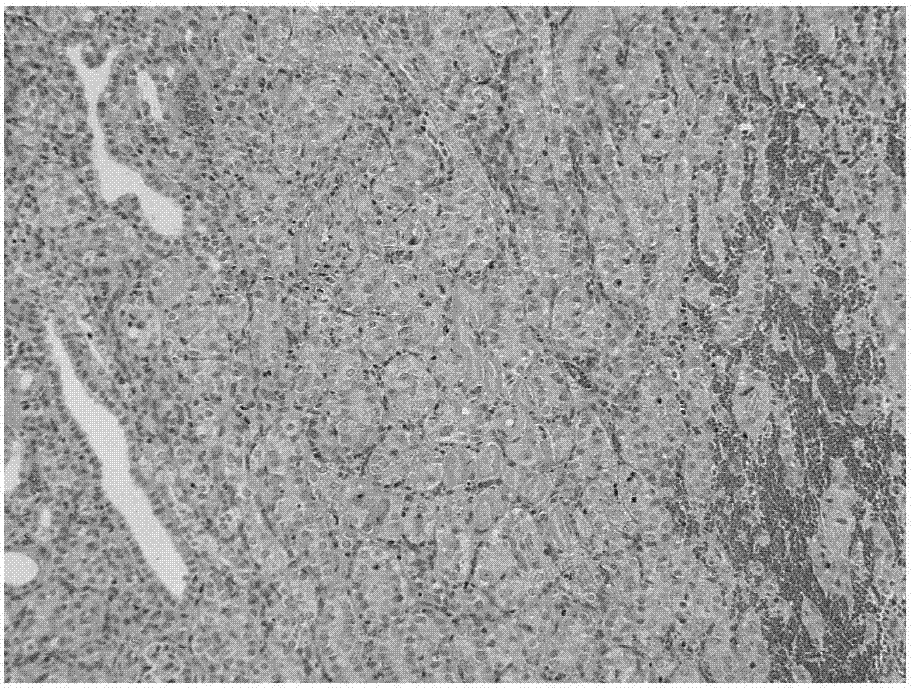
[0086] Example 3: Inhibitory effect of nintedanib ethanesulfonate on renal fibrosis in rats
[0087] 50 male SD rats were randomly divided into 5 groups according to the principle of similar body weight, 10 rats in each group, namely the sham operation group, the model control group, the low dose group of the test product, the medium dose group of the test product and the high dose of the test product Group. Experimental animals were anesthetized on day 0 (D0) and laparotomy was performed in the middle, and the left kidney was separated. The ureter was separated at the lower pole of the left kidney, and unilateral ureteral ligation (UUO) was performed. In the sham operation group, only laparotomy and separation of the left kidney were performed.
[0088] Dosing began on the next day (D1) after the operation, the animals in the sham operation group (group 1) were not treated, the animals in the model control group (group 2) were given vehicle, and the low dose group of the te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com