A preparation method of high-purity birnessite-type manganese oxide that efficiently degrades organic dyes
A technology of pure birnessite and organic dyes, applied in chemical instruments and methods, water pollutants, manganese oxide/manganese hydroxide, etc., can solve time-consuming and labor-intensive problems, limit the practical application of birnessite, long reaction time, etc. problem, to achieve significant effect, time-saving and labor-saving large-scale production, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] First, weigh 1.2g NaOH and dissolve in 50mL deionized water; secondly, accurately weigh 1.352g (8mmol) MnSO 4 ·H 2 O, 2.992g (8mmol) EDTA-Na was dissolved in 25ml of deionized water; under rapid stirring, NaOH solution was added dropwise to MnSO at 3mL / min 4 In the mixed solution with EDTA-Na, after the dropwise addition, continue to stir and age for 2 hours, filter and wash the precipitate several times, and then place it in a vacuum drying oven at 40°C for 12 hours to obtain high-purity birnessite.
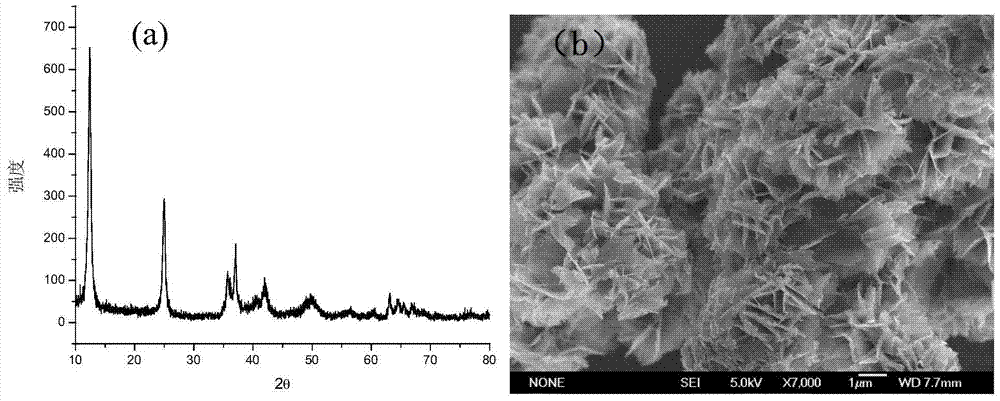
[0030] Carry out XRD detection to the prepared sample, such as figure 1 Shown in (a), product is high-purity birnessite, and crystallinity is higher, figure 1 (b) Its FESEM map. Accurately weigh 10mg of the sample and add it to a 50ml 10mg / L RhB volumetric flask, ultrasonically disperse the sample in the solution for 5 minutes, and then use H 2 SO 4 Adjust the pH of the solution to 1, take 3 mL samples at 1, 3, 5 and 10 minutes, centrifuge to get the supernatant, mea...
Embodiment 2
[0032] First, weigh 2.4g NaOH and dissolve in 50mL deionized water; secondly, accurately weigh 1.352g (8mmol) MnSO 4 ·H 2 O, 2.992g (8mmol) EDTA-Na was dissolved in 25ml of deionized water; under rapid stirring, NaOH solution was added dropwise to MnSO at 6mL / min 4 In the mixed solution with EDTA-Na, after the dropwise addition, continue to stir and age for 2 hours, filter and wash the precipitate several times, and then place it in a vacuum drying oven at 40°C for 12 hours to obtain high-purity birnessite.
Embodiment 3
[0034] First, weigh 1.2g NaOH and dissolve in 50mL deionized water; secondly, accurately weigh 2.704g (16mmol) MnSO 4 ·H 2 O, 4.984g (16mmol) EDTA-Na was dissolved in 100ml deionized water; under rapid stirring, NaOH solution was added dropwise to MnSO at 6mL / min 4 In the mixed solution with EDTA-Na, after the dropwise addition, continue to stir and age for 2 hours, filter and wash the precipitate several times, and then place it in a vacuum drying oven at 80°C for 6 hours to obtain a high-purity birnessite sample.
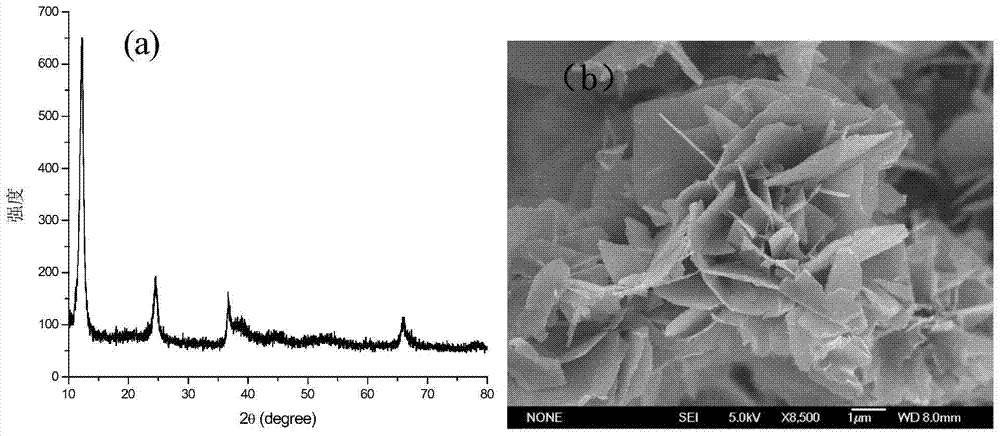
[0035] Carry out XRD detection to the prepared sample, such as figure 2 Shown in (a), product is high-purity birnessite, and crystallinity is higher, figure 2 (b) Its FESEM map. Accurately weigh 10mg of the sample and add it to a 50ml 10mg / L MB (methylene blue) volumetric flask, ultrasonically disperse the sample in the solution for 5min, and then use H 2 SO 4 Adjust the pH of the solution to 1, take 3 mL samples at 1, 3, 5 and 10 minutes, centrifuge to get...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com