Method for preparing polyester by ring-opening polymerization
A technology of ring-opening polymerization and polyester, applied in the field of organometallic catalyzed polymer materials, can solve problems such as being unsuitable for industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
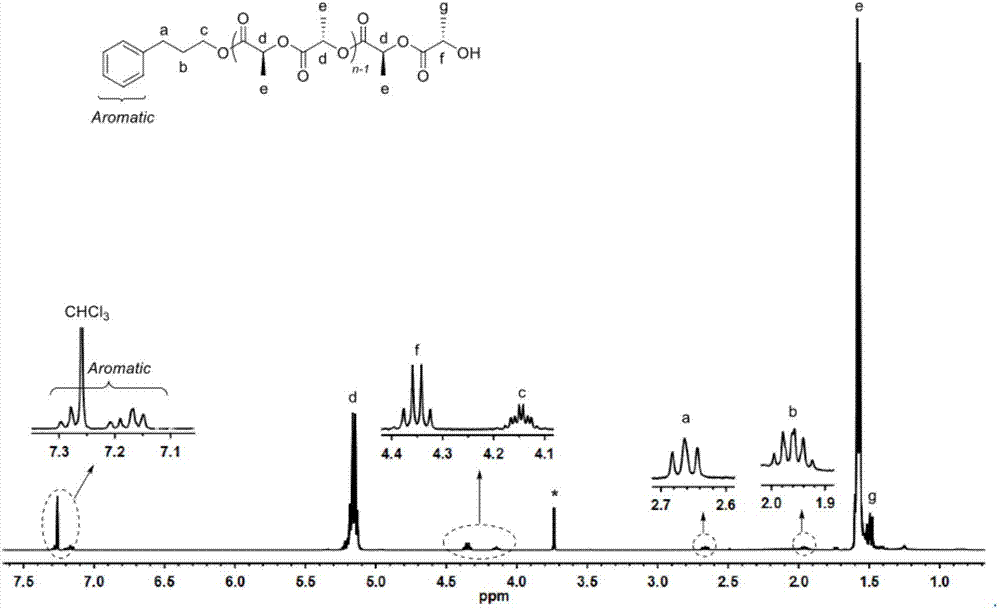
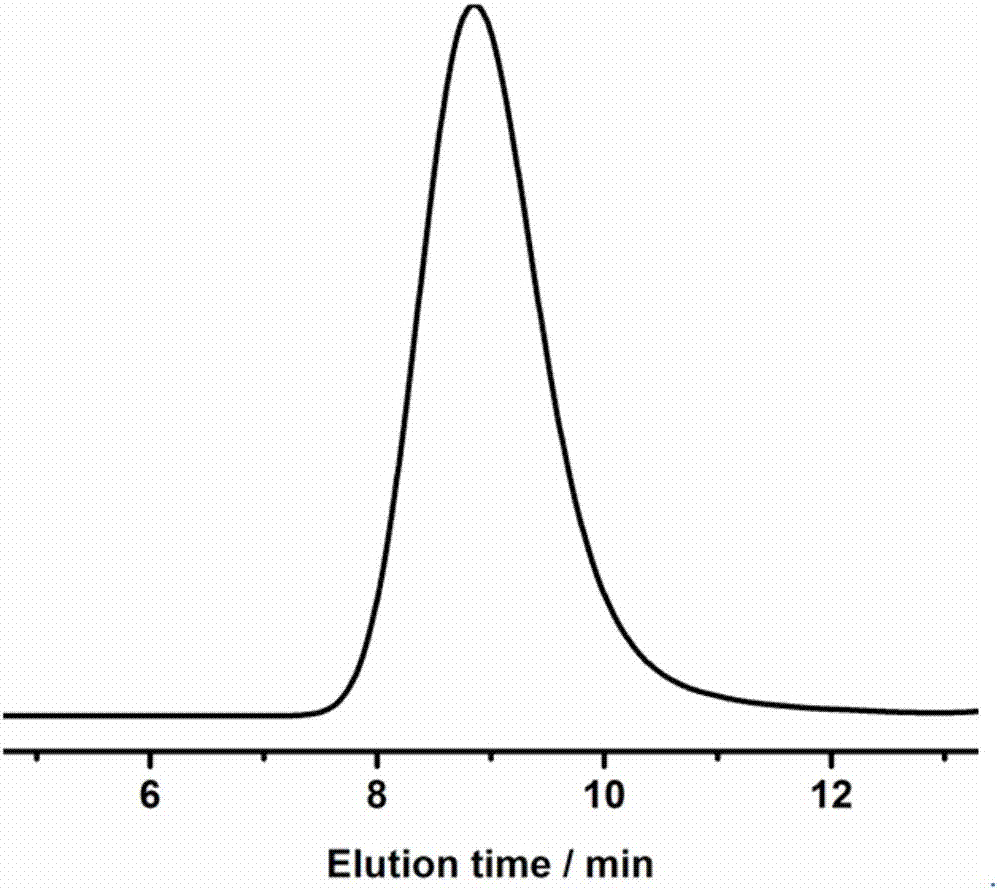
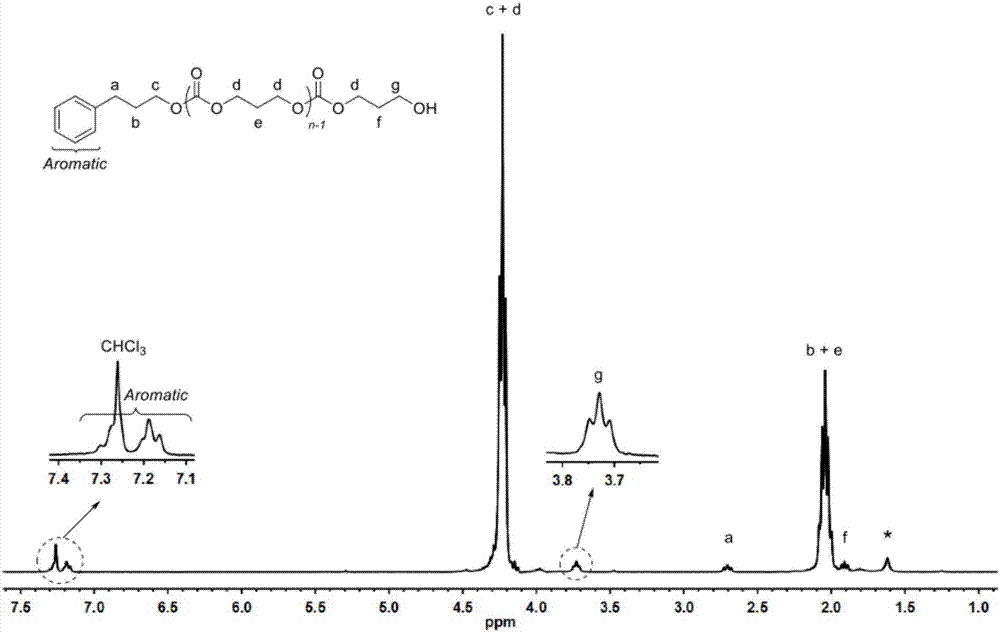
[0072] In a 10mL polymerization tube, add L-lactide (0.432g, 3mmol), compound (1) (0.022g, 0.1mmol), phenylpropanol (13.5μL, 0.1mmol), and stir magnetically at 130°C After 24 hours, the reaction was stopped, and a small amount of dichloromethane was added dropwise to the resulting mixture to dissolve, and then the resulting solution was slowly dropped into cold methanol, and a white polymer was precipitated. After centrifugation and vacuum drying, 0.38 g of the product was obtained, and the conversion rate was 98.7%. Number average molecular weight M of poly L-lactide n It is 4560g / mol, and the molecular weight distribution PDI is 1.18. (attached figure 1 , 2 )
Embodiment 2
[0074] In a 10mL polymerization tube, add D-lactide (0.432g, 3mmol), compound (8) (0.027g, 0.1mmol), pentaerythritol (9.7μL, 0.1mmol), and stir magnetically at 130°C for 20 hours , stop the reaction, drop a small amount of dichloromethane into the resulting mixture to dissolve, then slowly drop the resulting solution into cold methanol, a white polymer is precipitated, and obtain 0.29g of the product through centrifugation and vacuum drying, with a conversion rate of 96.1%. - Number average molecular weight M of lactide n It is 4610 g / mol, and the molecular weight distribution PDI is 1.19.
Embodiment 3
[0076] In a 10mL polymerization tube, add L-lactide (0.432g, 3mmol), compound (9) (0.058g, 0.1mmol), benzyl alcohol (10.0μL, 0.1mmol), and stir magnetically at 130°C for 18 After hours, the reaction was stopped, and a small amount of dichloromethane was added dropwise to dissolve the resulting mixture, and then the resulting solution was slowly dripped into cold methanol, and a white polymer was precipitated. After centrifugation and vacuum drying, 0.36g of the product was obtained, and the conversion rate was 98.4%. Number average molecular weight M of L-lactide n It is 4710g / mol, and the molecular weight distribution PDI is 1.18.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com