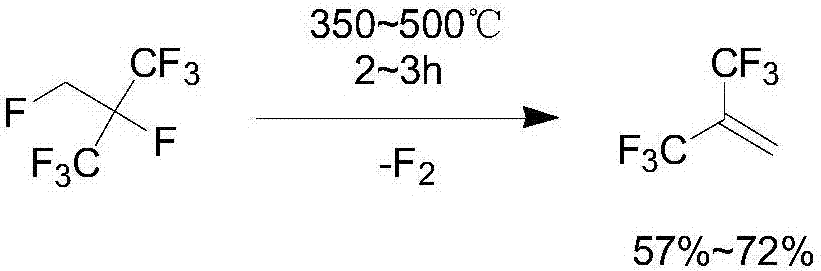
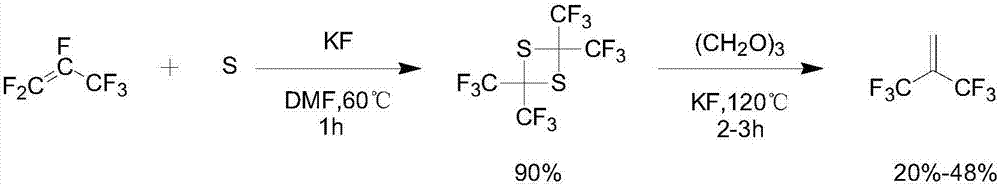
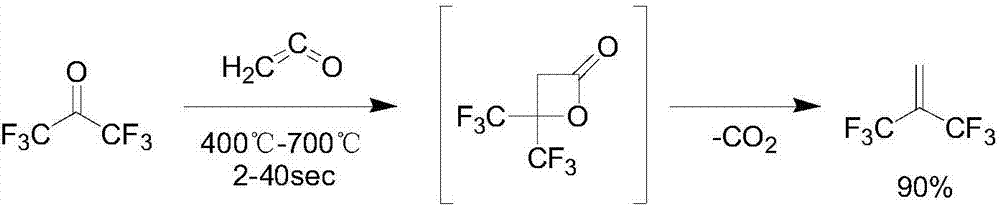
Synthesis method of hexafluoroisobutene
A technology for the synthesis of hexafluoroisobutene, which is applied in chemical instruments and methods, carbon-based compound preparation, hydroxyl compound preparation, etc., can solve the problems of unavailable raw materials, long routes, unfavorable industrialization, etc., and achieve resource utilization and separation The effect of simple purification and easy industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~4
[0048] In a 250ml three-necked round-bottomed flask, add 50ml of anhydrous methanol as a solvent, borohydride, and under stirring, dropwise add 106g (0.5mol) of heptafluoroisobutenyl methyl ether and 50ml of anhydrous methanol to react, and filter after the reaction. Distillation in hexafluoroisobutenyl methyl ether. The test results of other different conditions are shown in Table 1. Wherein, conversion rate and selectivity are based on heptafluoroisobutenyl methyl ether.
[0049] Table 1 Embodiment 1~4 hexafluoroisobutenyl methyl ether synthetic test result
[0050]
Embodiment 5~7
[0052] In a 250ml three-neck round bottom flask, add 50ml of solvent water, NaBH 4 9.5g (0.25mol) as a phase transfer catalyst, 106g (0.5mol) of heptafluoroisobutenyl methyl ether was added dropwise under stirring to start the reaction, after the reaction was completed, filtered and rectified to obtain hexafluoroisobutenyl methyl ether. The test results of other different conditions are shown in Table 2. Wherein, the amount of phase transfer catalyst, conversion rate and selectivity are all based on heptafluoroisobutenyl methyl ether.
[0053] Table 2 Embodiment 5~7 hexafluoroisobutenyl methyl ether synthetic test result
[0054]
Embodiment 8
[0056] In a 250ml three-neck round bottom flask, add 0.5mol of hexafluoroisobutenyl methyl ether prepared in Example 1, 2.0mol of sulfuric acid with a concentration of 98% by mass, stir evenly, react at 75°C for 4h, and distill out hexafluoroisobutenyl methyl ether under reduced pressure. Isobutyraldehyde, the yield is 92%, and the conversion rate is 100%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| critical temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com