Primer group and probe group capable of simultaneously detecting various influenza viruses and kit capable of detecting various influenza viruses
A technology for influenza virus and influenza A virus, applied in the field of molecular biology, can solve problems such as lack of detection of various influenza viruses, and achieve the effects of eliminating nucleic acid extraction steps, improving efficiency and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
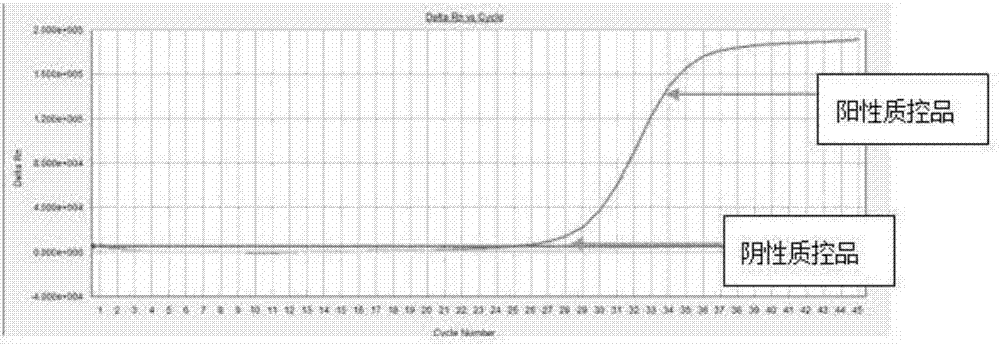
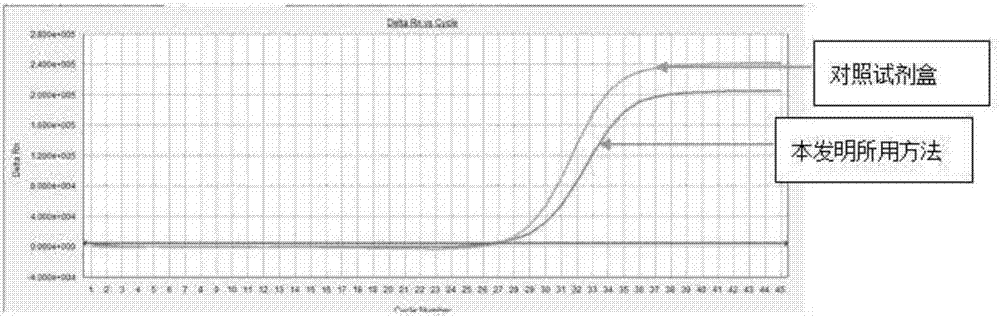
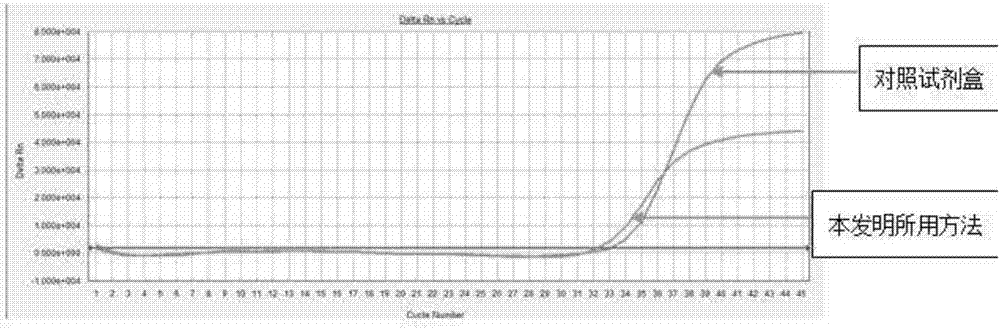
Image
Examples
Embodiment 1
[0034] For influenza virus IVA, IVB, H1 and H3, design specific primers respectively, and the primer sequences are as follows:
[0035] IVA-F: 5'-CTTCTAAGCGAGGTCGAACC-3'
[0036] IVA-R: 5'-GTCTTAGCCATTCGATGAGT-3'
[0037] IVB-F: 5'-CGACATCCAAAGCCAATTCG-3'
[0038] IVB-R: 5'-CGGTGCTCTTGACCAAATTGC-3'
[0039] H1-F: 5'-CCTCAGGGAGCTATAAACAGA-3'
[0040] H1-R: 5'-TGACCTACTTTGGACACTCTCC-3'
[0041] H3-F: 5'-CAACTGCAATTCTGAATGCATC-3'
[0042] H3-R: 5'-GATCCTGTTTACATTTTGGAATG-3'
[0043] For each set of primers, design its specific probe, the sequence is as follows:
[0044] IVAP: 5’-CAGGCCCCCTCAAAGCCG-3’ (5’ FAM; 3’ BHQ1)
[0045] IVBP: 5'-AGACTCCCACCGCAGTTTCAGC-3' (5' HEX; 3' BHQ1)
[0046]H1P: 5'-CCTTTCCAGAATGTACACCCAGTC-3' (5' Texas Red; 3' BHQ1)
[0047] H3P: 5'-CCAAATGGAAGCATTCCCAATGAC-3' (5' Cy5; 3' BHQ1)
[0048] The RT-PCR reaction system is: 28 μl of RT-PCR reaction solution A, 2 μl of RT-PCR reaction solution B, and 20 μl of sample eluent.
[0049] The RT-PCR rea...
Embodiment 2
[0083] Composition and preparation of embodiment 2 kit
[0084] A kit for simultaneously detecting multiple influenza viruses, comprising: direct RT-PCR reaction solution A, direct RT-PCR reaction solution B, sample diluent, positive quality control substance, and negative quality control substance.
[0085] The RT-PCR reaction solution A consists of: the four influenza virus-specific primers and probe sequences described in Example 1 (the primers are all 15 pmol, and the probes are all 10 pmol), 10 mM Tris-HCl (pH7.5), 2mM MgCl 2 , 0.2% (V / V) NP-40, 0.01% (V / V) Tween20, 30mM KCl, and the rest is DEPC water.
[0086] The RT-PCR reaction solution B consists of: reverse transcriptase (2.5U / reaction), Taq DNA polymerase (5U / reaction), dNTPs (0.6mm / reaction), RNase inhibitor (20U / reaction).
[0087] The sample diluent consists of: 10mM Tris-HCl (pH8.5), 0.2mM EDTA solution.
[0088] The composition of the positive quality control product is as follows: it consists of a plasmid ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com