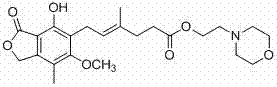
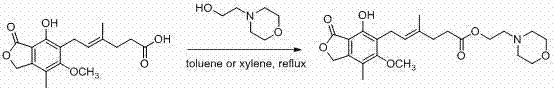
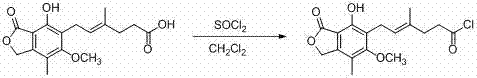
Preparation method of mycophenolate mofetil
A technology of mycophenolate mofetil and mycophenolic acid, which is applied in the field of preparation of mycophenolate mofetil, can solve the problems of "three wastes" difficult to handle, residual reaction raw materials, and many impurities, so as to avoid the generation of acid gas , the effect of mild reaction conditions and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Under the protection of nitrogen, Ph 3 P (32.8g, 125mmol, 1.25eq), morpholine ethanol (13.1g, 100mmol, 1.0eq) dissolved in 200ml of dichloromethane, cooled to 0℃, controlled the temperature not to exceed 5℃, add DIAD within 20-30min (28.3g, 140mmol, 1.4eq) in dichloromethane (40ml) solution, after the addition, the yellow solution was stirred for 10 minutes. Then add a solution of mycophenolic acid (33.6g, 105mmol, 1.05eq) in dichloromethane (50ml) at 0~5℃, after the addition, stir at room temperature for 4h. The reaction solution was poured into 200 ml of water, stirred for 5 minutes, and separated. The organic phase was extracted once with 120 ml of 1N hydrochloric acid solution. The hydrochloric acid solution was washed once with 50 ml of ethyl acetate, and the pH was adjusted to 8 with saturated sodium bicarbonate solution. Many white turbidity precipitated, followed by extraction with 80ml of dichloromethane and 30ml of dichloromethane, combined the organic phases, ...
Embodiment 2
[0038] Under the protection of nitrogen, Ph 3 P (18.4g, 70mmol, 1.40eq), morpholine ethanol (6.6g, 50mmol, 1.0eq) dissolved in 200ml ethyl acetate, cooled to 0℃, controlled the temperature not to exceed 10℃, add DIAD within 15-20min (15.2g, 75mmol, 1.50eq) in ethyl acetate (30ml) solution. After the addition, the orange solution was stirred for 15 minutes. Then add a solution of mycophenolic acid (17.6g, 55mmol, 1.10eq) in ethyl acetate (40ml) at 0~10℃, after the addition, stir at room temperature for 6h. Pour the reaction solution into 150ml of water, stir for 5min, separate the layers, extract the organic phase once with 60ml of 1N hydrochloric acid solution, then wash the hydrochloric acid solution with 30ml ethyl acetate once, adjust the pH to 8 with saturated sodium bicarbonate solution, there are more White turbidity precipitated, and it was extracted with 50ml ethyl acetate and 20ml ethyl acetate successively. The organic phases were combined, dried over anhydrous sodium...
Embodiment 3
[0041] Under the protection of nitrogen, Ph 3 P (68.2g, 260mmol, 1.30eq), morpholine ethanol (26.2g, 200mmol, 1.0eq) were dissolved in 2-methyltetrahydrofuran (500ml), then cooled to 5℃, controlled temperature not to exceed 10℃, Add DEAD (48.8g, 280mmol, 1.40eq) in 2-methyltetrahydrofuran (80ml) solution within 30min. After the addition, the orange solution is stirred for 15min. Then add a solution of mycophenolic acid (76.9g, 240mmol, 1.20eq) in 2-methyltetrahydrofuran (150ml) at 0~10℃. After the addition, stir at room temperature for 6h. Pour the reaction solution into 400ml water, stir for 5min, separate the layers, extract the organic phase once with 240ml of 1N hydrochloric acid solution, then wash the hydrochloric acid solution with 90ml ethyl acetate once, and adjust the pH to 9 with saturated sodium bicarbonate solution. White turbidity precipitated, and it was extracted with 100ml 2-methyltetrahydrofuran and 50ml 2-methyltetrahydrofuran successively, and the organic ph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com