Preparation and application of il7r gene deletion zebrafish mutant
A gene deletion, zebrafish technology, applied in the field of molecular biology, to achieve the effect of convenient in vivo experimental research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Screening for differentially expressed genes in a demyelination zebrafish model
[0049] 1. Steps
[0050] Using a gene chip (Affymetrix GeneChip Zeb Gene 1.0ST Array), the gene expression profile of the demyelination zebrafish model (the construction method is the same as the patent document: ZL2014101092570) was detected and analyzed. The criteria for judging differentially expressed genes are: ≥2 times is up-regulated; ≤0.5 times is down-regulated. Chip analysis found 1364 differential genes, including 271 up-regulated genes and 1093 down-regulated genes ( Figure 4 ). Among the many down-regulated genes, it was found that the expression of IL7R was significantly down-regulated (foldchange=0.44, *p=0.0061888). Real-time fluorescent quantitative PCR (qRT-PCR) and Western blot (Westernblot, WB) were used for verification. The verification method is: the experimental group is 5dpf Tg(mbp:nfsB-egfp), treated with 0.2%DMSO-5mM metronidazole (mitronidazole, M...
Embodiment 2
[0051] Example 2 Screening of sgRNA
[0052] 1. Experimental animals
[0053] Wild-type zebrafish (TU strain) was cultured according to a standardized protocol, the water temperature was 28.5°C, and the light / dark cycle was 14h / 10h. Embryos were collected after spawning of adult zebrafish and cultured in E3 hatching solution. The number of hours of fertilization (hours post- Fertilization, hpf) or days post-fertilization (dpf) indicate the developmental stage of embryos and larvae.
[0054] 2. CRISPR / Cas9 gene knockout target site design
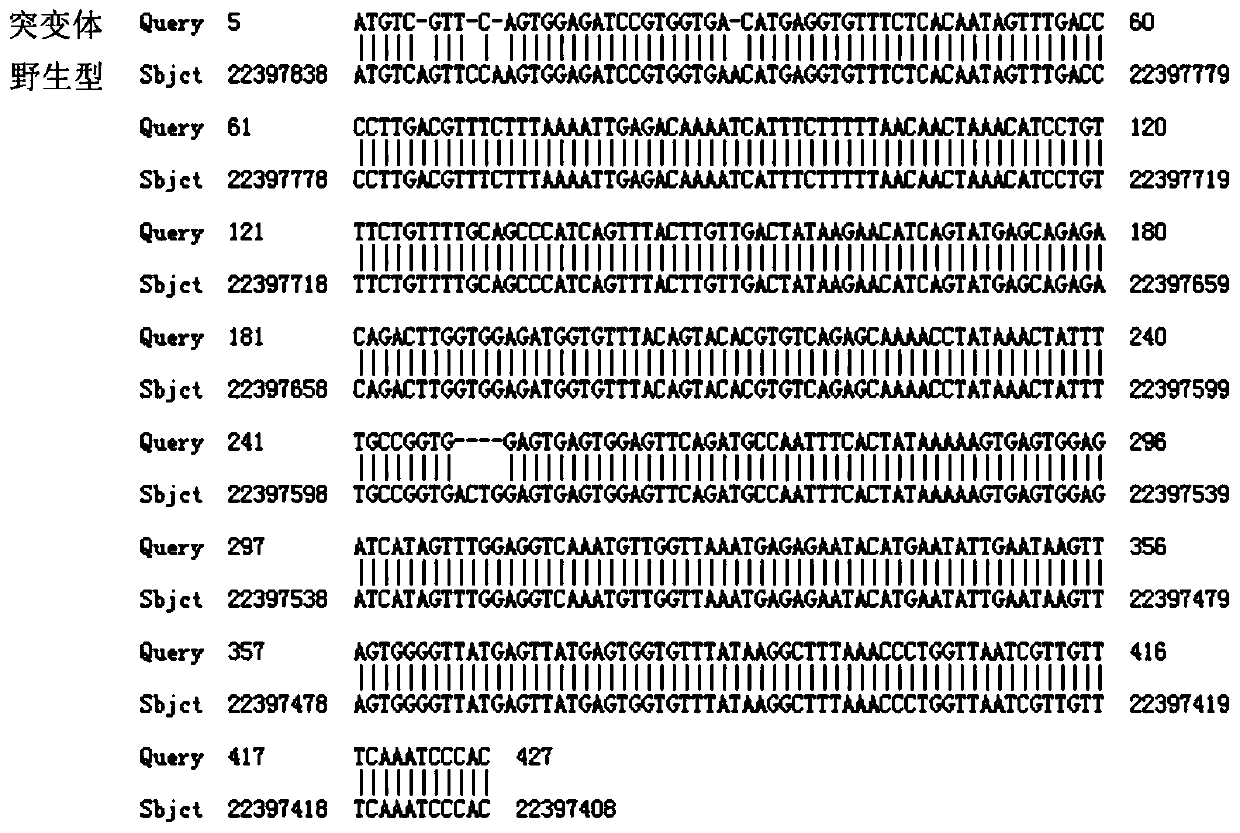
[0055] Query the genomic DNA sequence and functional domain of the zebrafish IL7R gene at http: / / asia.ensembl.org, and design a pair of IL7R at http: / / crispr.mit.edu / according to the principle of CRISPR / Cas9 knockout The target site of the gene, the target site contains 20 bases, the selection criteria of the target site is: 5'-GG-(N)20-NGG-3'; the GG dinucleotide at the 5' end is the T7 promoter The 3' end of the target site is NGG (N i...
Embodiment 3
[0063] Example 3 Microinjection of zebrafish embryos and determination of target site effectiveness
[0064] 1. sgRNA synthesis in vitro
[0065] 1.1PCR method to obtain the template for in vitro transcription of sgRNA
[0066] The forward primer sequence is: 5'-TAATACGACTCACTATAGACTCCACTCACTCCAGTCACgttttagagctagaaatagc-3' (the first 18 nucleotide sequences are the T7 promoter, the 19-38 nucleotide sequence is the sgRNA target sequence, and the nucleotide sequence represented by lowercase letters is the sgRNA upstream backbone); The reverse primer sequence is the downstream backbone of 25bp sgRNA, the sequence is 5'-AAAAAAAGCACCGACTCGGTGCCAC-3', and the amplification is carried out according to the following PCR conditions: pre-denaturation at 95°C for 3min; denaturation at 95°C for 30s, annealing at 54°C for 30s, extension at 72°C for 20s for 35 cycle, then 10min at 72°C;
[0067] 1.2 PCR product recovery and purification;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com