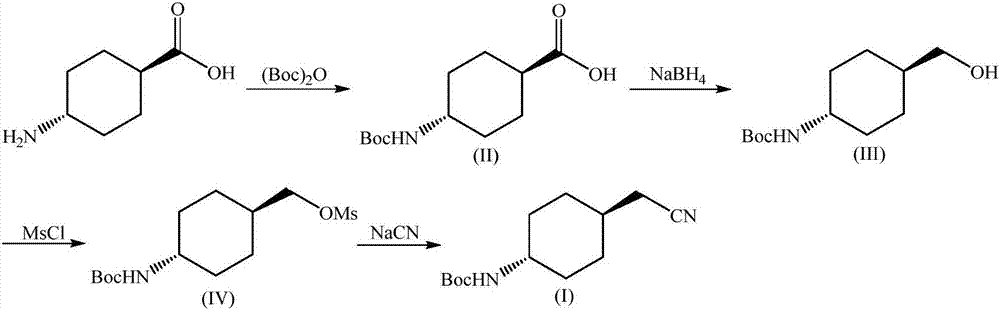
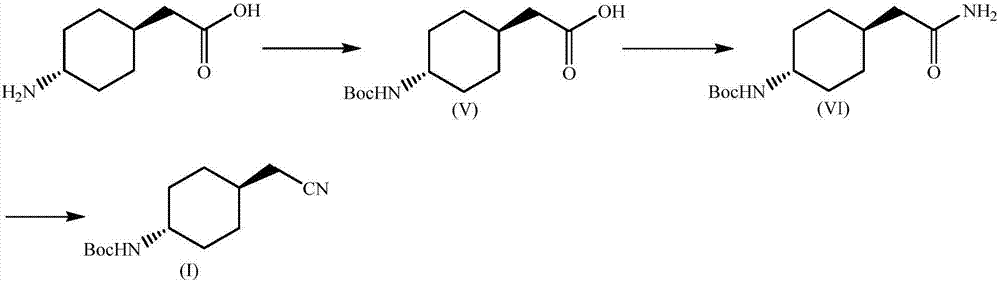
Preparation method for trans-(4-cyanomethyl)cyclohexyl tert-butyl carbamate
A technology of cyclohexyl carbamic acid and tert-butyl ester, which is applied to the preparation of carbamic acid derivatives, the preparation of organic compounds, chemical instruments and methods, etc. It can solve the problems of violent reaction, difficult control, flammability and explosion of sodium borohydride, etc. , to achieve the effect of high yield, short process route and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Boc anhydride is reacted with trans-(4-cyanomethyl)cyclohexylcarbamate tert-butyl ester to protect the amino group to generate the compound of formula (V). The compound of formula (V) (25.7 g, 0.10 mol) was dissolved in 150 mL of tetrahydrofuran, carbonyldiimidazole (21.1 g, 0.13 mol) was added at room temperature, stirred for 3 h, then concentrated ammonia water (30 mL) was slowly added dropwise, and stirred for 3 h. After the reaction, the solvent was removed under reduced pressure, and the residue was dissolved in ethyl acetate (200 mL), then washed with water (100 mL×2), washed with saturated brine (100 mL), dried over anhydrous sodium sulfate, and recovered under reduced pressure to obtain the formula (VI) compound. The yield was 92%.
[0027] The compound of formula (VI) (25.6g, 0.10mol) was dissolved in 200mL of tetrahydrofuran, cooled to 0°C, triethylamine (70mL, 0.50mol) was added, stirred for 10min, and then trifluoroacetic anhydride (35mL, 0.25 mol), keep s...
Embodiment 2
[0029] Boc anhydride is reacted with trans-(4-cyanomethyl)cyclohexylcarbamate tert-butyl ester to protect the amino group to generate the compound of formula (V). Dissolve the compound of formula (V) (25.7g, 0.10mol) in 150mL of dioxane, add carbonyldiimidazole (21.1g, 0.13mol) at room temperature, stir for 2h, then slowly drop concentrated ammonia water (30mL), stir 3h. After the reaction, the solvent was removed under reduced pressure, and the residue was dissolved in ethyl acetate (200 mL), then washed with water (100 mL×2), washed with saturated brine (100 mL), dried over anhydrous sodium sulfate, and recovered under reduced pressure to obtain the formula (VI) compound. The yield was 90%.
[0030] Dissolve the compound of formula (VI) (25.6g, 0.10mol) in 200mL of dichloromethane, cool to 0°C, add triethylamine (70mL, 0.50mol), stir for 10min, then slowly drop trifluoroacetic anhydride (35mL , 0.25mol), insulated and stirred for 3h. After the reaction, the organic phase...
Embodiment 3
[0032]Boc anhydride is reacted with trans-(4-cyanomethyl)cyclohexylcarbamate tert-butyl ester to protect the amino group to generate the compound of formula (V). The compound of formula (V) (25.7g, 0.10mol) was dissolved in 150mL of tetrahydrofuran, Boc anhydride (26.2g, 0.12mol) and pyridine (10mL, 0.124mol) were added at room temperature, stirred for 3h, and then concentrated ammonia water ( 30mL), stirred for 3h. After the reaction, the solvent was removed under reduced pressure, and the residue was dissolved in ethyl acetate (200 mL), then washed with water (100 mL×2), washed with saturated brine (100 mL), dried over anhydrous sodium sulfate, and recovered under reduced pressure to obtain the formula (VI) compound. The yield was 93%.
[0033] Dissolve the compound of formula (VI) (25.6g, 0.10mol) in 200mL of N,N-dimethylformamide, add pyridine (40mL, 0.50mol), stir at room temperature for 10min, then slowly drop phosphorus oxychloride (19mL , 0.20mol), stirred for 1h. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com