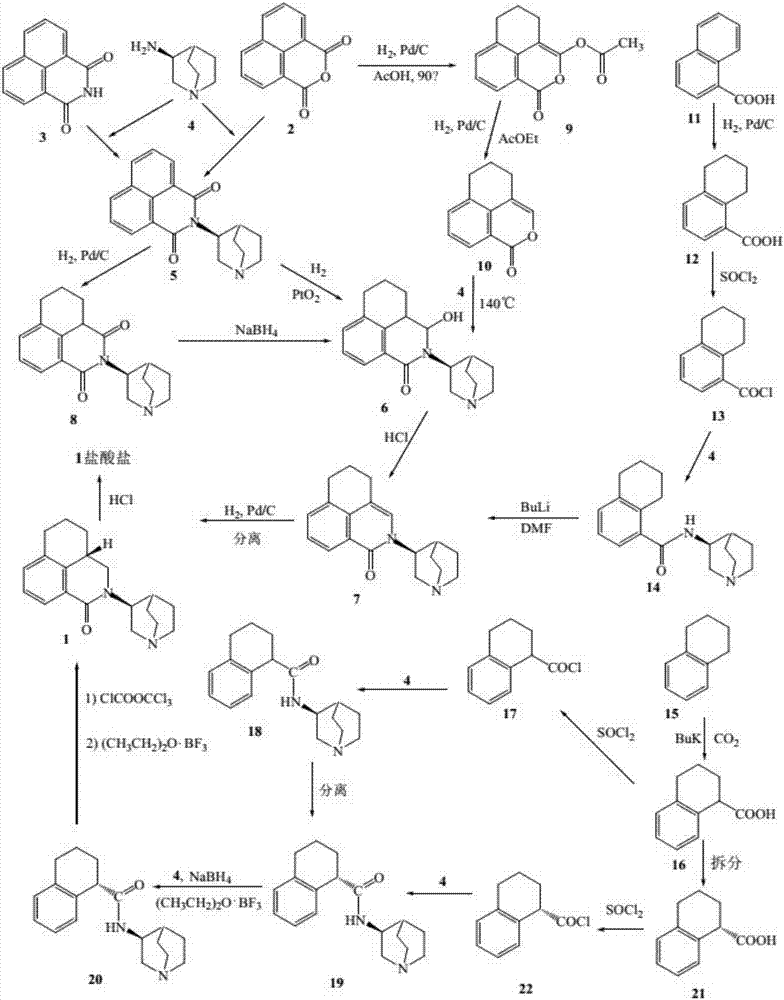
Synthesis process of palonosetron hydrochloride hydrochloride
A technology for palonosetron hydrochloride and a synthesis process, which is applied in the field of synthesis technology of palonosetron hydrochloride, can solve problems such as low yield, and achieve the effects of high yield and simplified steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
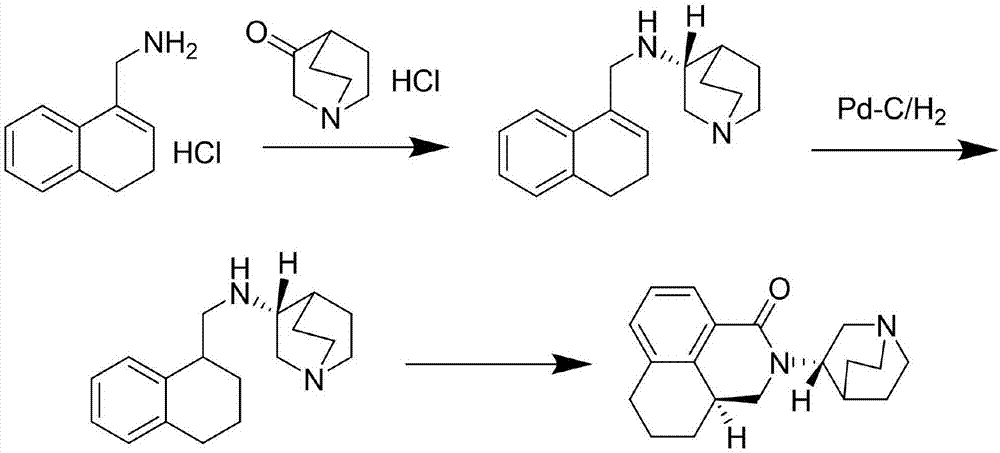
[0026] Example 1 N-[(3,4-dihydronaphthyl-1-yl )methyl]-(S)-1-azabicyclo[2.2.2]octane-3-amine n-butanol solution
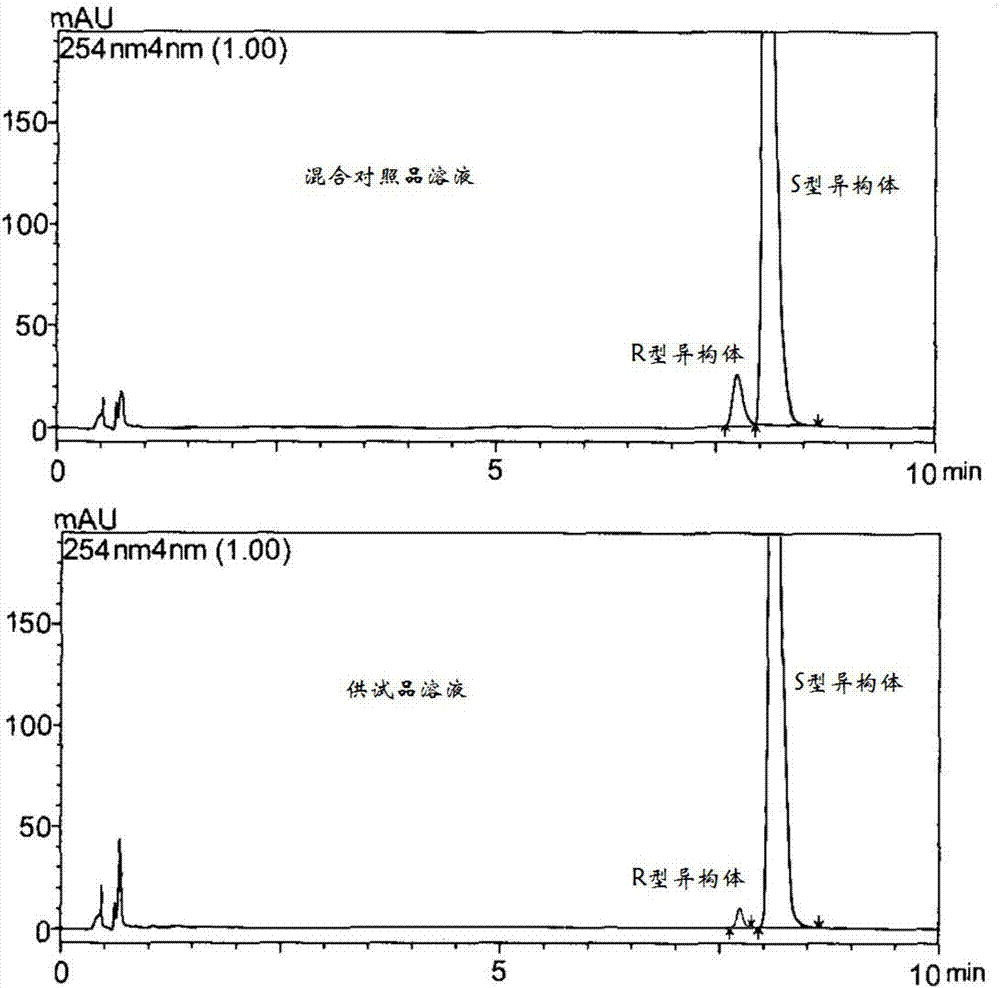
[0027] 3,4-dihydro-1-naphthylmethylamine hydrochloride (content greater than 99%, 35g, 0.18mol), 3-quinuclidone hydrochloride (content greater than 99%, 29g, 0.18mol) and toluene (300ml) was added triethylamine (45g, 0.45mol) under stirring, heated to reflux for 4h, the solvent was evaporated under reduced pressure, n-butanol (200ml) was added to the residue, cooled to 0°C, and sodium borohydride was added in batches (7.5g, 0.2mol), react at room temperature for 4h. Evaporate n-butanol under reduced pressure, add water (200ml) to the residue, extract with water-saturated n-butanol (200ml×2), combine organic phases, dry over anhydrous magnesium sulfate, filter to obtain N-[(3,4 -N-butanol solution of -dihydronaphthalen-1-yl)methyl]-(S)-1-azabicyclo[2.2.2]octane-3-amine (45g, yield 93.2%), take an appropriate amount For HPLC analysis. HPLC analysis method and ana...
Embodiment 2
[0050] Example 2 Directly in the n-butanol of N-[(3,4-dihydronaphthalene-1-yl)methyl]-(S)-1-azabicyclo[2.2.2]octane-3-amine Add catalyst to the solution for hydrogenation reduction to obtain N-[(1,2,3,4-tetrahydronaphthalene-1-yl)methyl]-(S)-1-azabicyclo[2.2.2]octane-3 -amine
[0051] Take an appropriate amount of N-[(3,4-dihydronaphthalene-1-yl)methyl]-(S)-1-azabicyclo[2.2.2]octane-3-amine in n-butanol and concentrate to About 200ml, containing N-[(3,4-dihydronaphthalene-1-yl)methyl]-(S)-1-azabicyclo[2.2.2]octane-3-amine 14g (0.05mol ), adding 5% Pd-C (2g), reacting with hydrogen at room temperature for 8h, filtering, and concentrating the filtrate to dryness under reduced pressure to obtain N-[(1,2,3,4-tetrahydronaphthalene-1-yl)methyl ]-(S)-1-Azabicyclo[2.2.2]octane-3-amine (14 g, yield 99.5%). 1 HNMR (CDCl 3 )δ:0.93(brs,1H),1.25-1.45(m,2H),1.55-1.65(m,2H),1.75-1.90(m,5H),2.41(d,J=13.4Hz,1H),2.68 -2.80(m,8H),2.84-2.92(m,2H),3.08-3.20(m,1H),7.05-7.25(m,4H).
Embodiment 3
[0052] Example 3 N-[(1,2,3,4-tetrahydronaphthalene-1-yl)methyl]-(S)-1-azabicyclo[2.2.2]octane-3-amine ring Synthetic salt to get palonosetron hydrochloride
[0053] N-[(1,2,3,4-tetrahydronaphthalen-1-yl)methyl]-(S)-1-azabicyclo[2.2.2]octane-3-amine (14g, 0.05 mol) dissolved in toluene (100ml), added triphosgene (6g, 0.02mol) at room temperature, stirred for 8h, added 46% boron trifluoride ether solution (15ml), heated to reflux for 5h, added 1mol / L hydrochloric acid (60ml ), cooled to 10°C after reflux for 1h, adjusted to pH 9 with 30% sodium hydroxide solution (about 35ml), extracted the aqueous phase with ethyl acetate (50ml×2), combined the organic phases, dried over anhydrous magnesium sulfate, and filtered , the filtrate was concentrated to dryness under reduced pressure, isopropanol (50ml) and concentrated hydrochloric acid (4g, 0.04mol) were added to the remaining light yellow syrup, stirred and crystallized, filtered, the filter cake was recrystallized with isopropano...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com