Trans-N-glycosidase BtNGT and application thereof
A glycosyltransferase and glycosylation technology, applied in the field of N-glycosyltransferase BtNGT, can solve the problems of complicated steps and high cost, and achieve the effect of simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: Preparation of N-glycosyltransferase BtNGT
[0027] 1. Construction of expression strains
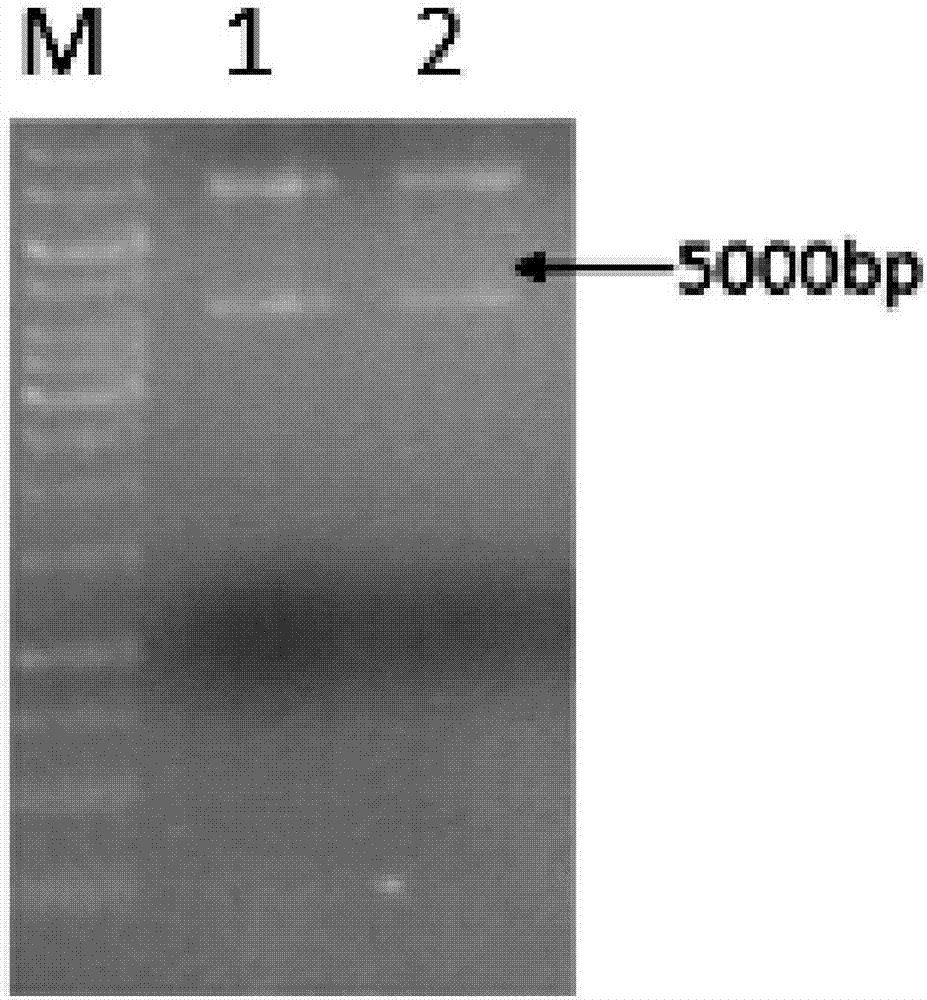
[0028] Transform the plasmid synthesized by GenScript into BL21 competent cells, heat shock at 42°C for 1 min, and spread on Amp plate. Pick a single colony into a 5ml test tube culture medium at 37°C and 200rpm for 12h, and verify the plasmid. The results of plasmid agarose gel electrophoresis figure 1 .
[0029] 2. Expression and purification of BtNGT protein
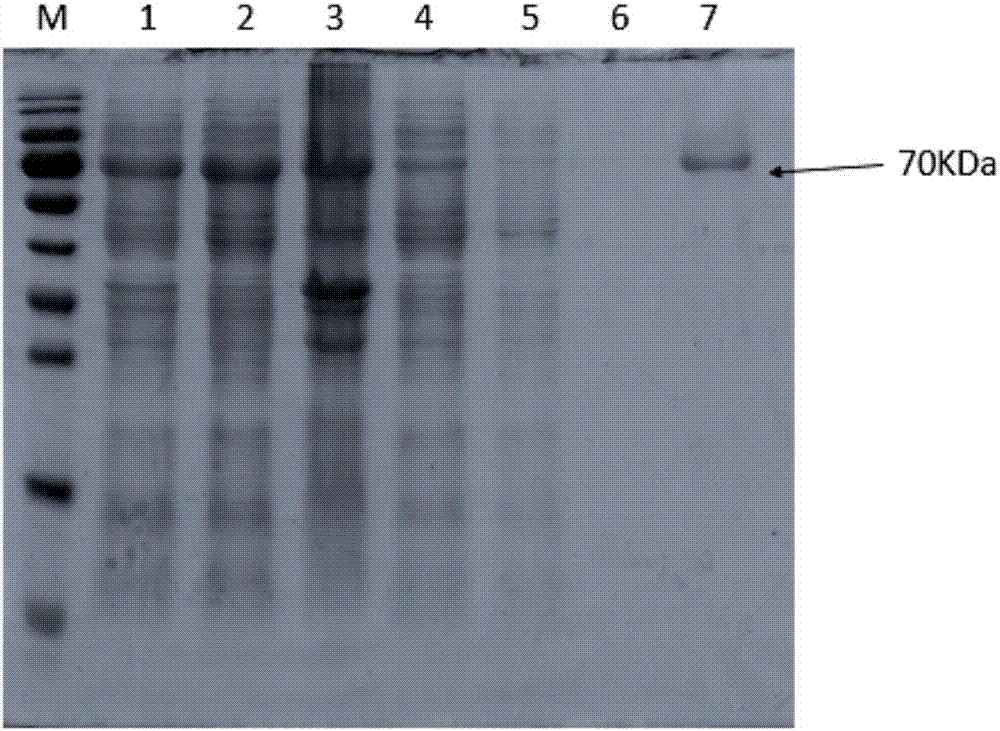
[0030] A single colony BL21pET45b-BtNGT was picked into 50ml LB medium (containing 50ug / ml ampicillin Amp) and activated at 200rpm at 37°C for 12h. Then expand the culture, transfer the bacterial solution to 1L culture medium (containing 50ug / ml ampicillin Amp), incubate at 200rpm at 37°C for about 3.5h, measure the OD value, when the OD 600 When the value is 0.6, ice-bath for 20 minutes, and then add 400 μL of 0.5 M IPTG to induce protein expression (16° C. 200 rpm). After 20 hours of induction, coll...
Embodiment 2
[0033] Example 2: Application of N-glycosyltransferase BtNGT in polypeptide glycosylation modification
[0034] 1. Determination of enzyme activity:
[0035] The substrate peptide for the determination of enzyme activity is the fluorescently labeled hexapeptide DANYTK synthesized by Nanjing GenScript Company. Under the catalysis of NGT, it reacts with UDP-Glc or UDP-Gal respectively. The reaction solution is detected by HPLC fluorescence detector. The system is shown in Table 1-3:
[0036] Table 1-3 AaNGT and BtNGT enzyme activity detection reaction system
[0037]
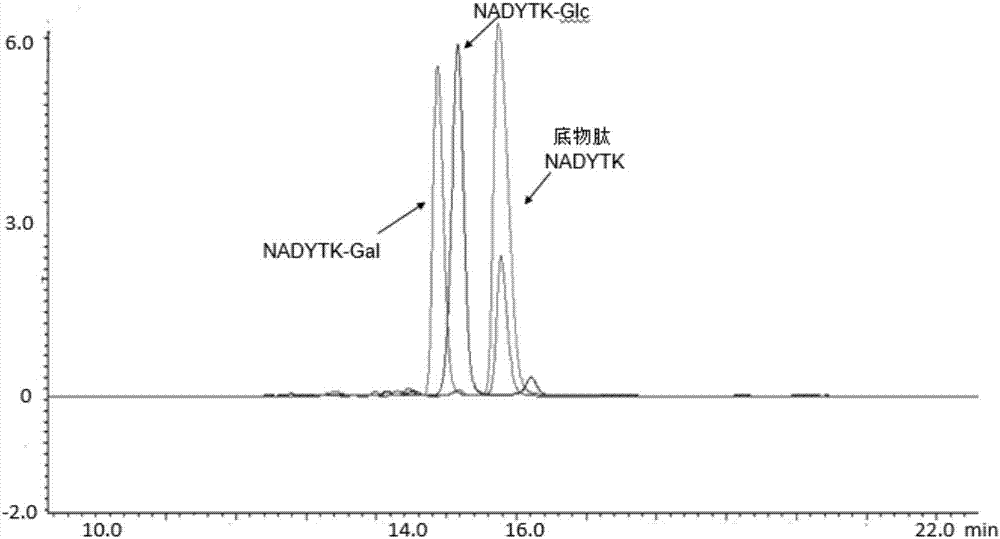
[0038] See the reaction result image 3 , the experiment proves that BtNGT can use UDP-Glc and UDP-Gal to complete the glycosylation modification of polypeptides. Then carry out simple verification on the product, see the simple result picture Figure 4 .
[0039] 2. Determination of optimum pH:
[0040] In order to explore the optimal pH of BtNGT, we selected buffers with different pH, HAc (5.0, 6.0) PBS...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com