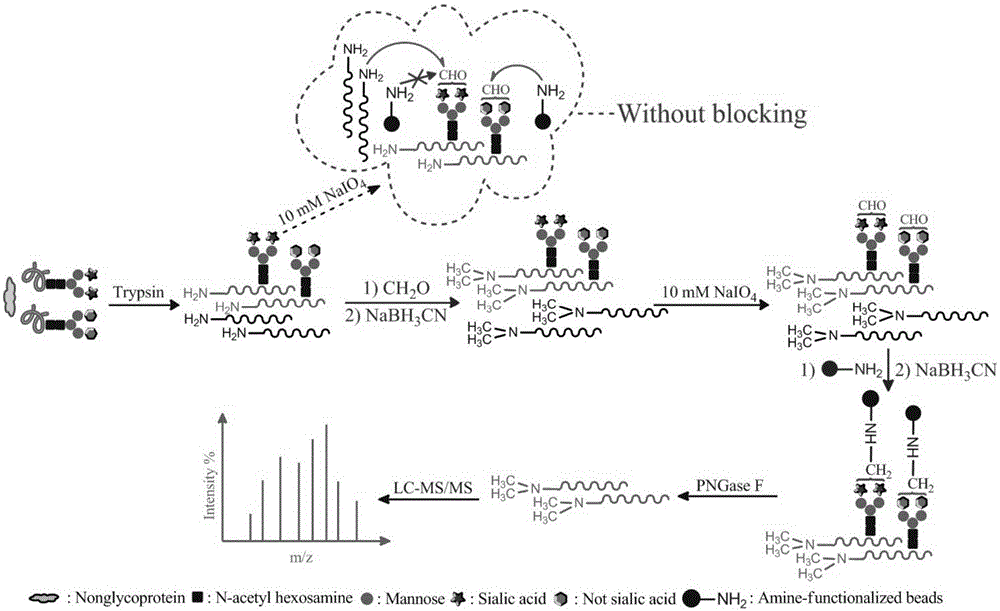
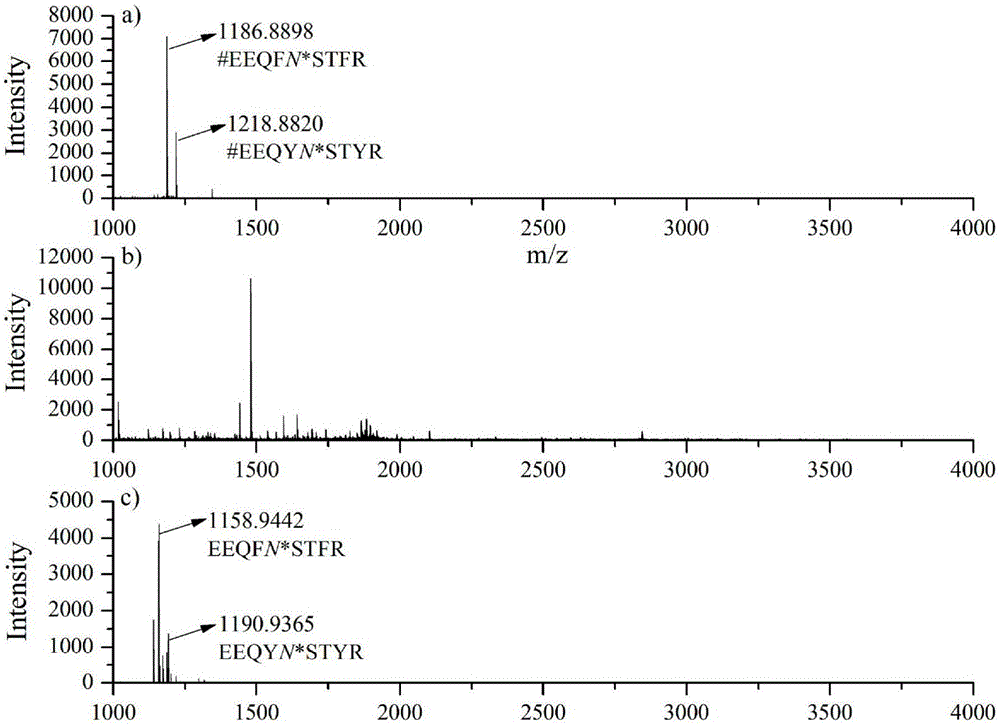
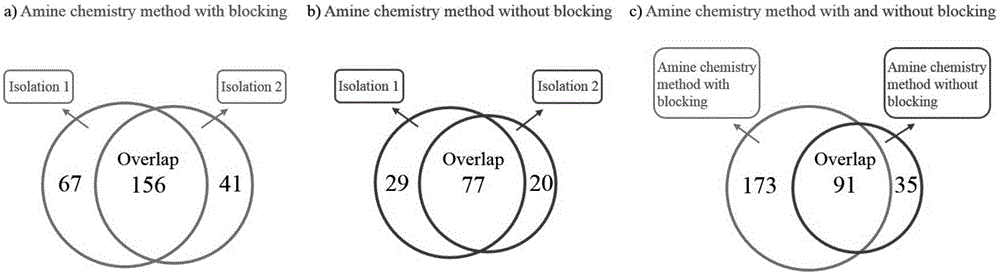
Method for selective enrichment and identification of N-linked glycopeptide
A sugar-linking and selective technology, applied in the field of selective enrichment of N-linked glycopeptides, can solve the problems of reducing the efficiency of amino chemical methods and the interference of amino microspheres enriching glycopeptides, achieving high enrichment specificity and improving Identify the effect of coverage and material availability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Verify the feasibility of the described amino chemistry based on two reductive amination reactions:
[0039] Human immunoglobulin (IgG) is a simple and easy-to-obtain glycoprotein, and bovine serum albumin (BSA) is a commonly used standard non-glycoprotein. N-glycopeptides in the peptide mixture of the separation of the two are enriched by using the amino chemical method based on two reductive amination reactions of the present invention. The experimental procedure is as follows:
[0040] 1. Dissolve 200 μg of IgG and BSA at a molar ratio of 1:1 in 96 μL of 100 mM TEAB buffer (pH 8.0), heat inactivate in boiling water for 10 min, cool to 25°C, add 4 μg of trypsin, and place in a 37°C water bath Medium enzymatic hydrolysis for 12h;
[0041] 2. Add 20 μL of 4wt% CH to the resulting peptide solution 2 O / H 2 O and freshly prepared 20 μL of 0.6M NaBH 3 CN aqueous solution, react in a shaker at 25°C for 1h;
[0042] 3. Add 30 μL of 8wt% ammonia water to quench the label...
Embodiment 2
[0050] We used the amino chemistry method based on two reductive amination reactions and the amino chemistry method based on one reductive amination reaction to analyze the N-glycoproteome information in human liver cancer serum, and compared the two methods to identify Results of N-glycosylation sites.
[0051] Human liver cancer serum used in this experiment was provided by the Second Affiliated Hospital of Dalian Medical University (Dalian, China). The acquisition and use of the samples were completely legal and complied with the relevant regulations of the ethics committee of the hospital. The experimental procedure for serum samples using amino chemistry based on two reductive amination reactions is as follows:
[0052] 1. Take 15 μL (containing about 1 mg of protein) serum, dilute it to 92 μL with a buffer (pH 8.0) containing 8M Urea and 100 mM TEAB, add 8 μL of 1M DTT, and react in a water bath at 60°C for 1 hour to open the disulfide bond, then Add 0.74 mg of IAA, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com