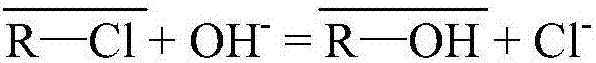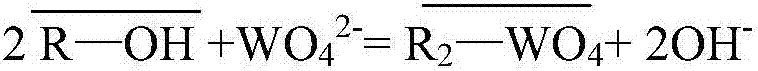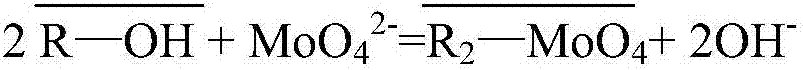Method for removing arsenic from nickel sulfate solution
A technology of nickel sulfate solution, which is applied in the field of industrial metallurgy, can solve the problems of loss of main metal elements, reduction of arsenic adsorption, etc., and achieve the effect of reducing dosage and improving conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Prepare 1L of nickel sulfate solution containing nickel 60g / L and arsenic (pentavalent) 50mg / L (AR grade nickel sulfate and arsenic acid, distilled water for water); (25°C) by WO 4 2 type resin, the WO 4 2 The type resin is obtained through the transformation of D301 type anion resin, and the arsenic in the above-mentioned ion-exchanged liquid is detected; then the exchanged D301 anion resin is rinsed with 0.2mol / L dilute sulfuric acid for nickel, and then the resin is washed to medium with distilled water. , and then use 0.1mol / L sodium hydroxide and 0.5mol / L sodium sulfide mixed solution to carry out arsenic removal treatment on the cleaned resin, so that arsenic is detached from the resin and enters the desorption solution, and the desorption solution is measured.
[0046] The concentration of arsenic in the solution after exchange is 0.142ppm, and the loss rate of tungsten is 0.0034%.
Embodiment 2
[0048] Prepare 1L of nickel sulfate solution containing 80g / L nickel and 50mg / L arsenic (pentavalent) (AR grade nickel sulfate and arsenic acid, distilled water for water); (40℃) by WO 4 2 type resin, the WO 4 2 The type resin is obtained through the transformation of D201 anion resin, and the arsenic in the above-mentioned ion-exchanged solution is detected; then the exchanged D201 anion resin is rinsed with 0.2mol / L dilute sulfuric acid for nickel, and then the resin is washed with distilled water until it is neutral. , and then use 0.1mol / L sodium hydroxide and 0.5mol / L sodium sulfide mixed solution to carry out arsenic removal treatment on the cleaned resin, so that arsenic is separated from the resin and enters the desorption solution, and the desorption solution is measured.
[0049] The concentration of arsenic in the solution after exchange is 0.216ppm, and the loss rate of tungsten is 0.0042%.
Embodiment 3
[0051] Prepare 1L of nickel sulfate solution containing 40g / L of nickel and 100mg / L of arsenic (pentavalent) (nickel sulfate and arsenic acid are of AR grade, and distilled water is used for water); (25°C) through MoO 4 2 type resin, the MoO 4 2 The type resin is obtained through the transformation of D301 anion resin, and the arsenic in the above-mentioned ion-exchanged solution is detected; then the exchanged D301 anion resin is rinsed with 0.2mol / L dilute sulfuric acid for nickel, and then the resin is washed with distilled water until it is neutral. , and then use 0.1mol / L sodium hydroxide and 0.5mol / L sodium sulfide mixed solution to carry out arsenic removal treatment on the cleaned resin, so that arsenic is separated from the resin and enters the desorption solution, and the desorption solution is measured.
[0052] The concentration of arsenic in the liquid after exchange is 0.194ppm, and the loss rate of molybdenum is 0.0016%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com