A benzene hydrogenation catalyst and a preparing method thereof
A catalyst and a technology for hydrogenation of benzene, which are applied in the direction of hydrogenation to hydrocarbons, chemical instruments and methods, and heterogeneous catalyst chemical elements, etc., can solve the problems of less industrial application of platinum-based catalysts, and achieve low prices and good active center dispersion performance , The effect of reducing the proportion of micropores
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] First, 8.0 g of the water-soluble chitosan pore-enlarging agent was added to deionized water at 50° C., and then acetic acid was added dropwise until the chitosan was completely dissolved to obtain an acid solution containing the pore-enlarging agent. Weigh a certain amount of tin nitrate, lanthanum nitrate and potassium carbonate respectively, completely dissolve tin nitrate, lanthanum nitrate and potassium carbonate in 70g of distilled water to form an aqueous solution containing tin, lanthanum and potassium. Weigh 350g of pseudo-boehmite powder and 20.0g of fennel powder into the kneader, and mix evenly, then add the mixed solution of tin nitrate, lanthanum nitrate and potassium carbonate, and finally add the acid solution containing chitosan to the pseudo-boehmite The boehmite powder is evenly kneaded, and then kneaded-extruded into a clover shape. Dry at 120° C. for 8 hours, and calcined at 700° C. for 4 hours to obtain an alumina carrier 1 containing tin, lanthanu...
Embodiment 2
[0032]Add 8.0 g of the water-soluble chitosan pore-enlarging agent into deionized water at 50° C., and then add acetic acid dropwise until the chitosan is completely dissolved to obtain an acid solution containing the pore-enlarging agent. Weigh a certain amount of tin nitrate, lanthanum nitrate and potassium carbonate respectively, completely dissolve tin nitrate, lanthanum nitrate and potassium carbonate in 70g of distilled water to form an aqueous solution containing tin, lanthanum and potassium. Weighing 350g pseudo-boehmite powder and 20.0g fenugreek powder join in the kneader, and mix evenly, then add the mixed solution of tin nitrate, lanthanum nitrate and salt of wormwood, finally the acid solution containing chitosan is added to The pseudo-boehmite powder is evenly kneaded, and then kneaded-extruded into a clover shape. Dry at 120° C. for 8 hours, and calcined at 700° C. for 4 hours to obtain an alumina carrier 2 containing tin, lanthanum and potassium. SnO in carrie...
Embodiment 3
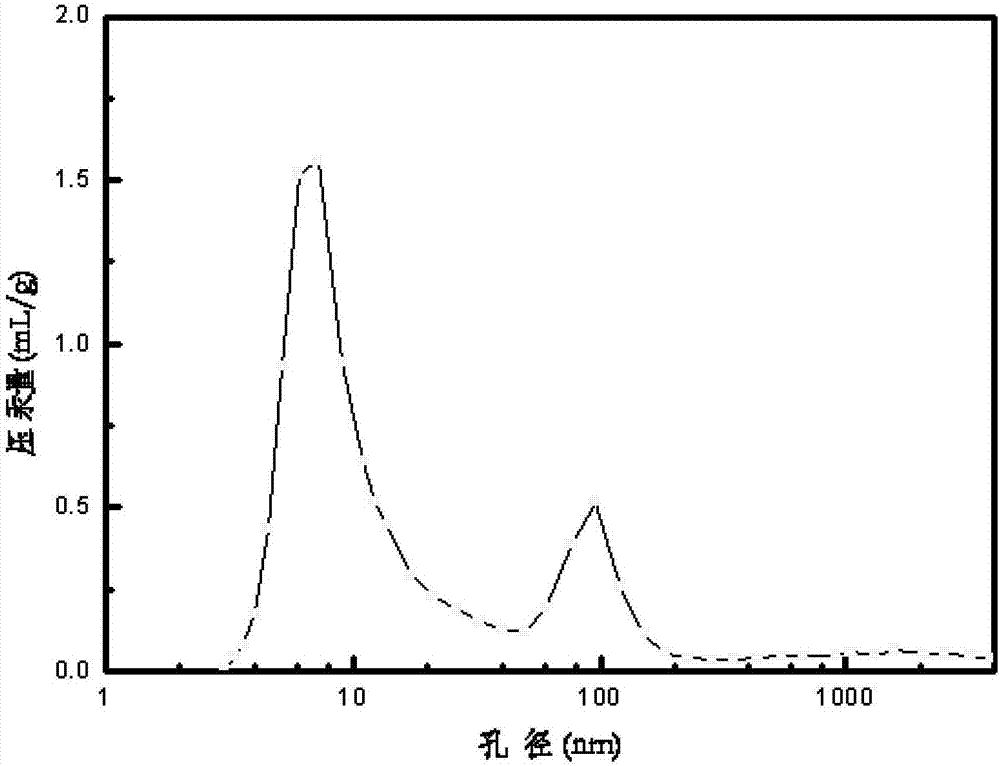
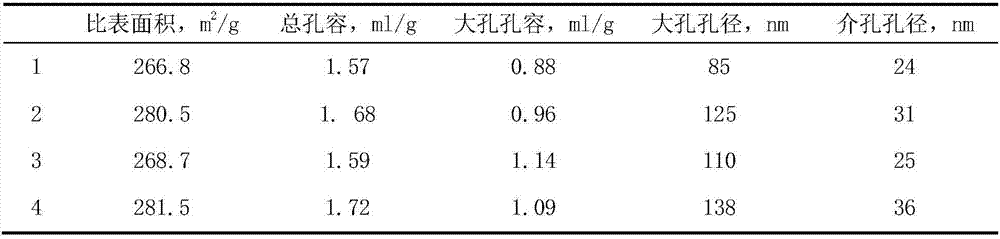
[0036] The preparation method of the carrier was carried out according to Example 1. The difference is that the water-soluble chitosan pore-enlarging agent is replaced by the non-water-soluble chitosan pore-enlarging agent, and the chitosan formic acid solution is stirred with a magnetic stirrer for 30 minutes to obtain the alumina carrier 3 with a macroporous structure. The content of auxiliary components tin, lanthanum and potassium in the carrier accounts for the percentage of the carrier mass, respectively SnO 2 1.5wt%, La 2 o 3 0.8wt% and K 2 O 2.8 wt%. Its specific surface area and pore size distribution are shown in Table 1.
[0037] Get chloroplatinic acid and palladium nitrate and join in 30ml distilled water, add ammoniacal liquor to adjust the pH value at 3.0, then dilute with deionized water, make impregnating liquid and impregnate spherical alumina carrier 100g with macroporous structure, the catalyst precursor that obtains is in After drying at 120°C for 6 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com