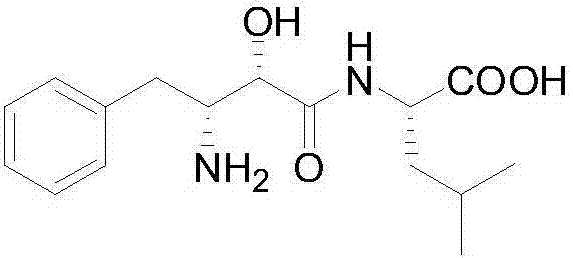
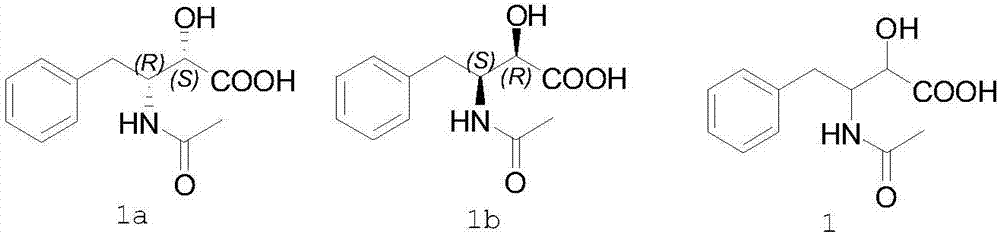
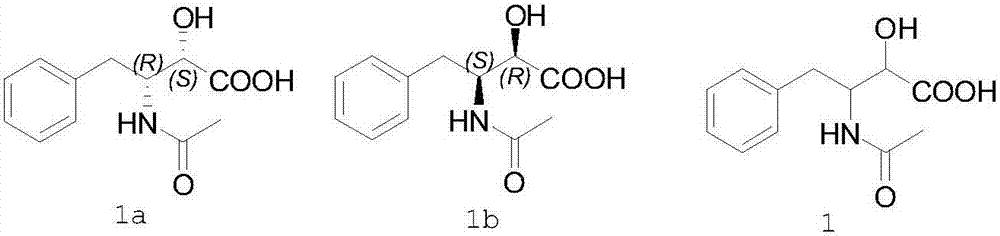
Preparation method of ubenimex intermediate (2S,3R)-3-acetamido-2-hydroxyl-4-phenylbutyric acid
A technology of acetamido and phenylbutyric acid, applied in the preparation of carboxylic acid amide optical isomers, organic chemistry, etc., can solve the problems of complicated operation, low yield, unfavorable industrial production, etc., and achieves easy operation and reproducibility Good, the effect of improving productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Compound 1 (2.4 g) and S-1-naphthylethylamine (1.8 g) were added into ethanol (10 ml), stirred and dissolved at 60°C. Cool the above solution to 25°C, filter to obtain 1.5 grams of white solid, which is the S-1-naphthylethylamine salt of (2S,3R)-3-acetamido-2-hydroxy-4-phenylbutyric acid. Add the salt to an aqueous solution (10 ml), stir to dissolve, add reagent hydrochloric acid (2 ml), stir to precipitate a solid, and separate to obtain (2S,3R)-3-acetylamino-2-hydroxy-4-phenylbutyric acid 0.85 g, yield 35.4%, ee value 99.8%.
Embodiment 2
[0033] Compound 1 (2.4 g) and S-1-naphthylethylamine (1.8 g) were added into ethanol (10 ml) and acetonitrile (3 ml), stirred at 60° C. to dissolve. Cool the above solution to 25°C, filter to obtain a white solid, which is the S-1-naphthylethylamine salt of (2S,3R)-3-acetamido-2-hydroxy-4-phenylbutyric acid, add this salt to into an aqueous solution (10 ml), stirred to dissolve, added reagent hydrochloric acid (2 ml), stirred to precipitate a solid, and separated to obtain 0.89 g of (2S,3R)-3-acetylamino-2-hydroxyl-4-phenylbutyric acid , yield 37.1%, ee value 99.6%.
Embodiment 3
[0035] Compound 1 (2.4 g) and (S)-1-(2-naphthyl)ethylamine (1.8 g) were added into tetrahydrofuran (20 ml), and stirred at 60°C to dissolve. Cool the above solution to 25°C, filter to obtain a white solid, which is the S-1-naphthylethylamine salt of (2S,3R)-3-acetamido-2-hydroxy-4-phenylbutyric acid, add this salt to into an aqueous solution (10 ml), stirred to dissolve, added reagent hydrochloric acid (2 ml), stirred to precipitate a solid, and separated to obtain 0.88 g of (2S,3R)-3-acetylamino-2-hydroxyl-4-phenylbutyric acid , yield 36.7%, ee value 99.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com