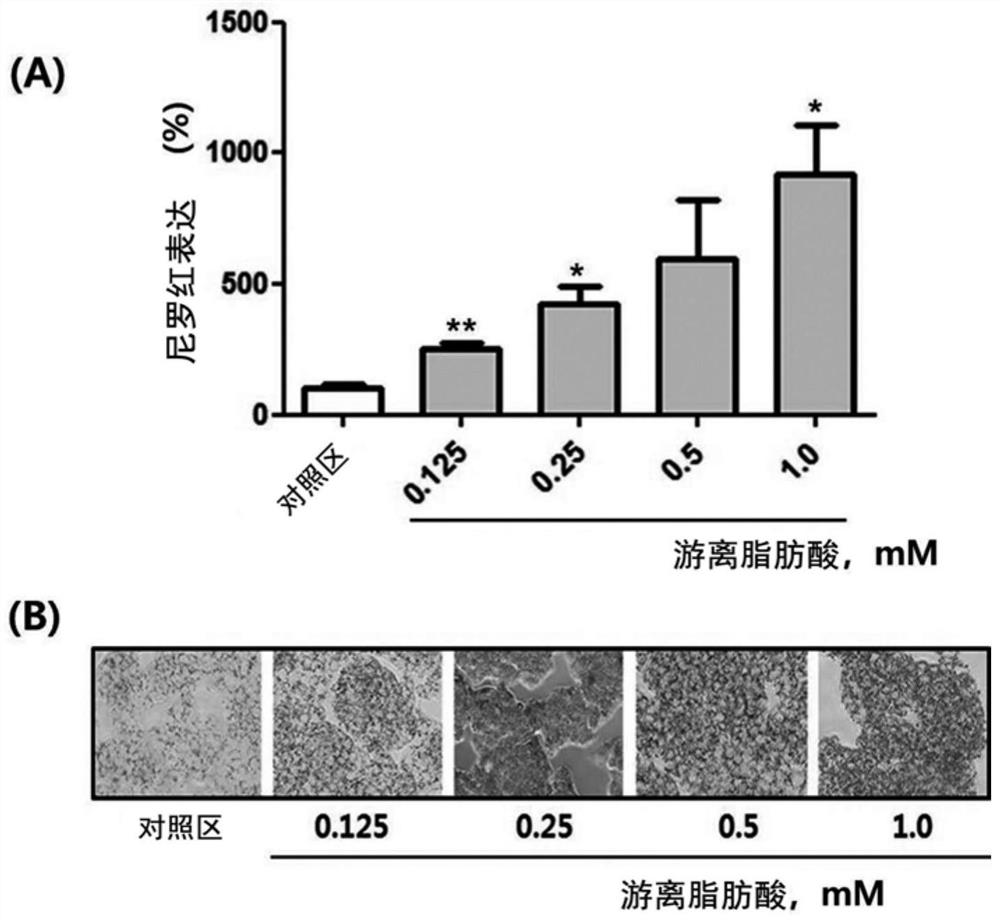
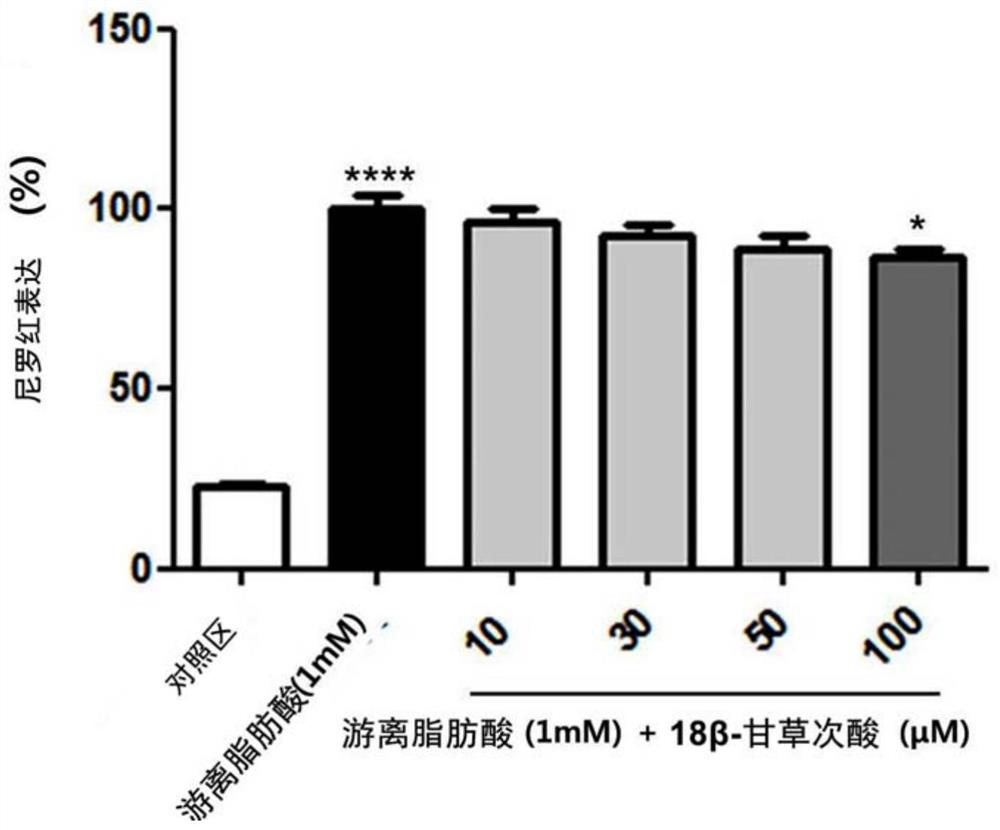
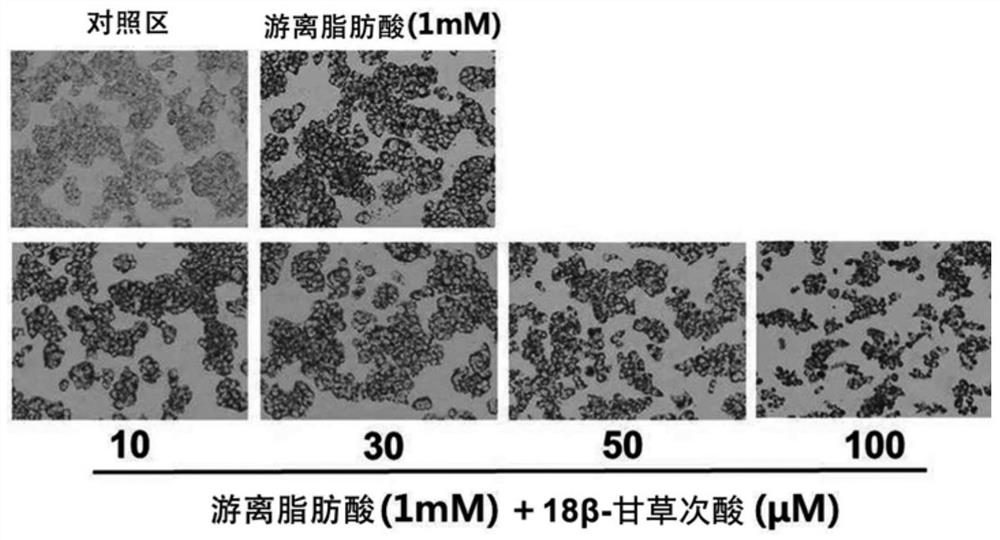
Composition for preventing or improving non-alcoholic fatty liver disease containing white lentil extract as an active ingredient
A non-alcoholic, active ingredient technology, applied in the field of health functional food composition and pharmaceutical composition, can solve the problems of non-alcoholic fatty liver efficacy research, increase of physiological activity, reporting, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0065] 2) Preparation of Oil Red O working solution
[0066] Mix 6 ml of the Oil Red O stock solution and 4 ml of ddH 2 After O was mixed, it was filtered through a 0.2 μm filter and left at room temperature for 20 minutes.
[0067] 3) Preparation of 10% formalin solution
[0068] ddH in 63ml 2 A stock solution (37%, Merck, Cat# K36658003) obtained by diluting 27ml of formalin stock solution in O was mixed with 10ml of 10XPBS.
[0069] 4) 100% isopropanol (Isopropanol; Merck, Cat# K36543834) was used as a blank control.
[0070] 5) Preparation of 60% isopropanol
[0071] Mix 6 ml of the 100% isopropanol and 4 ml of ddHO 2 O made by mixing.
Embodiment 1
[0072] Example 1. Liver cancer cell line (HepG 2 ) cultivation
[0073] Hepatoma cell line (HepG 2 ) was purchased from American Type Culture Collection (ATTC) and used. Hepatoma cell line (HepG 2 ) was cultured in a DMEM (Dulbecco's modified Eagle's medium) medium containing 10% FBS and 1% penicillin-streptomycin. Incubate the above cells at 37 °C in 5% CO 2 cultured in an incubator, and the cultured cells were cultured in a certain number (3×10 4 Cells / 500 μl well) were seeded into 24-well plates and experimented.
[0074] After the cells were seeded in the 24-well plate, the cells were completely attached to the 24-well plate and had the shape of the cells, and were used when the cells reached 75% confluence.
Embodiment 2
[0075] Embodiment 2. Preparation of free fatty acid (Free Fatty Acid; FFA)
[0076] (1) Preparation of 100mM palmitate (Sigma P-0500) and 100mM oleate (Sigma O-75010) stock solutions
[0077] It was prepared at 70° C. using a 0.1 M NaOH solution as a solvent, filtered through a 0.2 μm filter, and then sterilized.
[0078] (2) Preparation of 5% (w / v) BSA (Sigma A-6003) solution not containing free fatty acids
[0079] distilled water (ddH 2 O) It was prepared for use as a solvent, sterilized, and refrigerated at 4°C.
[0080] (3) The concentration of oleic acid ester and palmitic acid ester is the preparation of the 5mM mixed fatty acid stock solution that mixes at 2:1
[0081] At 60°C, the 5% (w / v) BSA solution that did not contain free fatty acids dissolved in distilled water prepared in (2) above was used as a solvent to prepare 10 ml of a fatty acid stock solution, so that the above (1) The concentrations of the respective fatty acids prepared in 2.0 were up to 5 mM. F...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com