Ocular delivery of drugs
A drug and composition technology, applied in the field of eye delivery of drugs, can solve problems such as limited shelf life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0091] Materials and methods
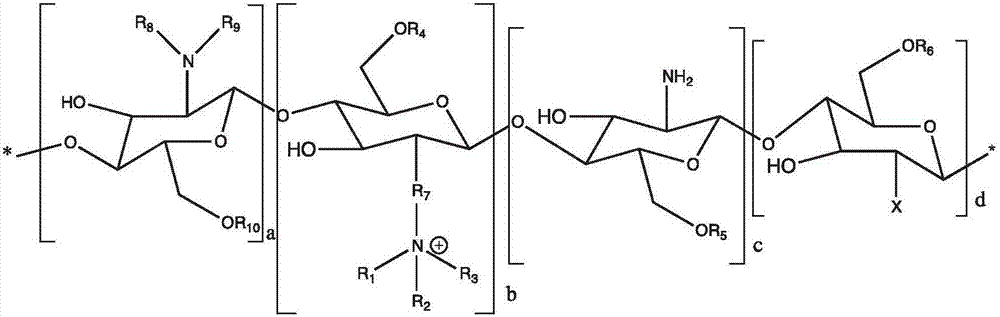
[0092] polymer
[0093] N-palmitoyl-N-monomethyl-N,N-dimethyl -N,N,N-Trimethyl-6-O-ethylene glycol chitosan (GCPQ). The GCPQ used in the experiment contained 20.51 Mol% palmitoyl / monomer unit, 11.93 Mol% quaternary ammonium group / monomer unit, and a molecular weight of 9.13 kDa.
[0094] CSA composition
[0095] Compositions comprising CSA were prepared as follows. Phosphate buffered saline (pH=7.4, 20 mL) was added to the weighed polymer sample and the weighed drug sample. The initial polymer / drug weight ratio was 7.5:1, and the drug content was adjusted to concentrations of 0.05%, 0.08%, and 0.1% w / v. The liquid mixture was vortexed for 2 minutes to ensure complete mixing, followed by high pressure homogenization (Avestin Emulsiflex, GCT Technology, UK) at 20,000 psi for 30 cycles.
[0096] CsA preparation stability
[0097] For stability analysis, aliquots of the formulation were stored in triplicate at refrigerated (2-3°C), room temper...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com