A chiral organic dye molecule with blue fluorescence and its preparation method and application
A technology of chiral resolution and organic solvents, applied in the field of fluorescent materials, can solve the problems of low efficiency and few types of blue light materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
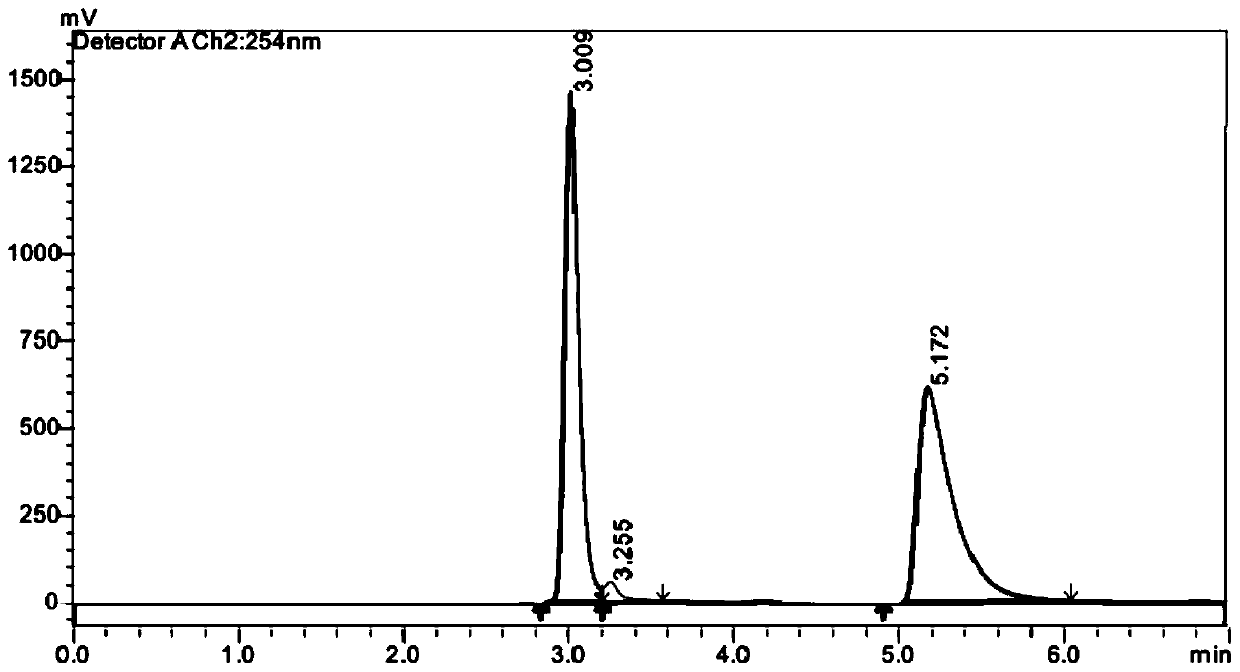
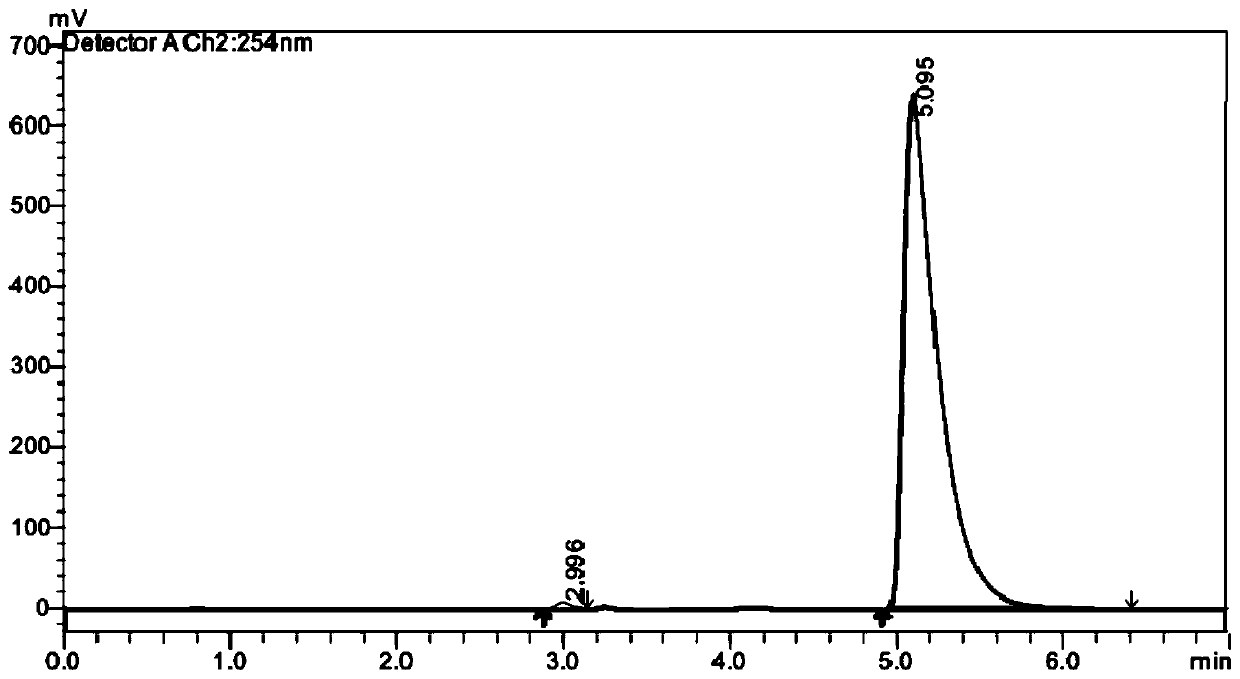
[0087] Embodiment 1, compound shown in preparation formula (M)-1A and formula (P)-1A
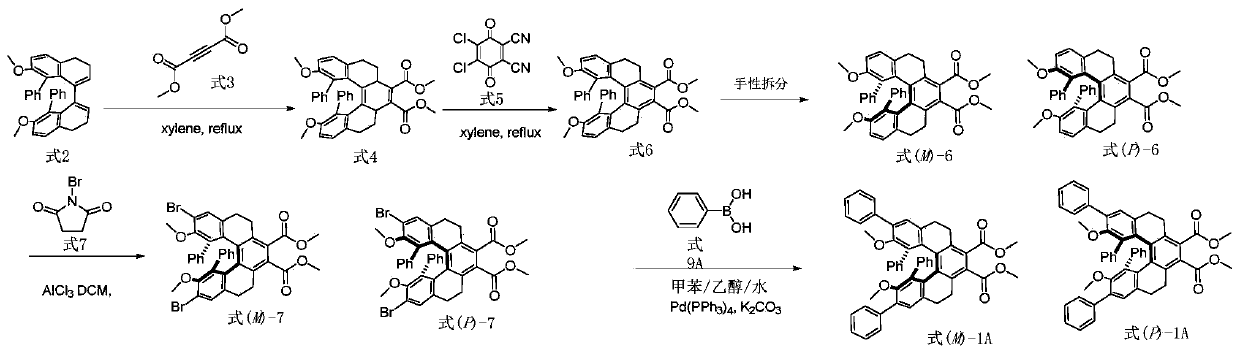
[0088] according to figure 1 The compound shown in the synthetic route preparation formula (M)-1A and formula (P)-1A, concrete steps are as follows:
[0089] 1) Add 50g of 2,2'-diphenyl-7,7'-dimethoxy-3,4,3',4'-tetrahydro-1,1'-dimethoxy to a 1000ml round bottom flask Naphthalene (structural formula as shown in formula 2), 150g methyl butynedicarboxylate (structural formula as shown in formula 3) and 500ml xylene were heated to reflux for 10 hours, cooled, spin-dried reaction solution, separated and purified through chromatographic column to obtain 61.9 The compound shown in g addition product formula 4 has a yield of 95%; the structural verification data are as follows: 1 H NMR (500MHz, CDCl 3 ): δ7.10(tt,J=7.1,1.3Hz,2H),6.99(td,J=7.2,1.5Hz,2H),7.09(d,J=6.0Hz,4H),6.86 (s,2H) ,6.77(s,2H),6.14(d,J=7.6Hz,2H),3.89(s,6H),2.97(s,6H),2.69–2.61(m, 2H),2.75(td,J=15.4 ,3.9Hz,2H),2.65(t,J=15.0,4.2...
Embodiment 2
[0103] Embodiment 2, compound shown in preparation formula (M)-1B and formula (P)-1B
[0104] The preparation method is the same as in Example 1, only the phenylboronic acid in step 4) is replaced with 595 mg of 4-methylphenylboronic acid (the structural formula is shown in formula 9B), and the synthetic route is as follows Figure 7 As shown, 244 mg of the compound represented by (M)-1B and formula (P)-1B was obtained, and the yield was 74%. The structure verification data is as follows: 1 H NMR (500MHz, CDCl 3 )δ7.59(d,J=7.7Hz,4H), 7.27–7.24(m,4H),6.99(t,J=7.4Hz,2H),6.93(s,4H),6.85(d,J=6.3 Hz, 4H), 6.23(d, J=7.6Hz, 2H), 3.91(s, 6H), 2.71(s, 6H), 2.70–2.65(m, 2H), 2.48(dd, J=15.3, 4.0Hz , 2H),2.43(s,6H),2.22(dd,J=13.7,3.2Hz,2H),1.42(td,J=14.8,4.0Hz,2H).HRMS (APCI)MS:791.3367([M+ H] + ). For related spectra, see Figure 8 and Figure 9 . Verified that the structure is correct.
Embodiment 3
[0105] Embodiment 3, compound shown in preparation formula (M)-1C and formula (P)-1C
[0106] The preparation method is the same as in Example 1, only the phenylboronic acid in step 4) is replaced by 610 mg of 4-methoxyphenylboronic acid (structural formula shown in formula 9C), and the synthetic route is as follows Figure 10 As shown, 251 mg of the compound represented by (M)-1C and formula (P)-1C was obtained with a yield of 75%. The structure verification data is as follows: 1 H NMR (500MHz, CDCl 3 )δ7.64(s,2H),7.62(s,2H),7.02–6.98(m,6H),6.93(d,J=4.9Hz,4H),6.89–6.81(m,4H),6.23(d ,J=7.6 Hz,2H),3.91(s,6H),3.88(s,6H),2.71(s,6H),2.68(d,J=3.4Hz,2H),2.46(td,J=15.4, 4.0Hz, 2H), 2.22(dt, J=13.9, 3.1Hz, 2H), 1.42(td, J=14.7, 4.0Hz, 2H).HRMS(APCI) MS: 823.3265([M+H] + ). For related spectra, see Figure 11 and Figure 12 . Verified that the structure is correct.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com