Method for synthesizing prohexadione calcium and trinexapac-ethyl
A synthesis method and procyclonic acid calcium technology are applied in chemical instruments and methods, preparation of carboxylic acid esters, preparation of organic compounds, etc., which can solve the problems of long reaction time, increased system pressure, and increased risk, and achieve synthesis conditions. Simple, simple and safe operation, convenient for industrial amplification effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
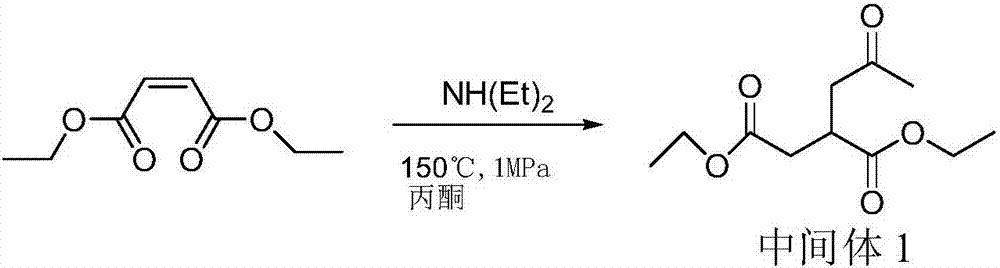
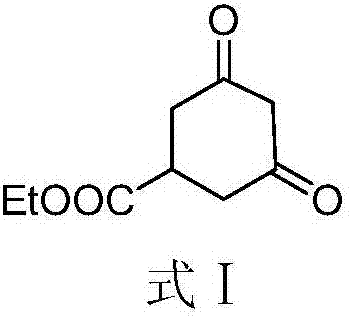
[0043] Embodiment 1, the synthesis of prohexadione calcium
[0044] Carry out according to following reaction equation:
[0045]
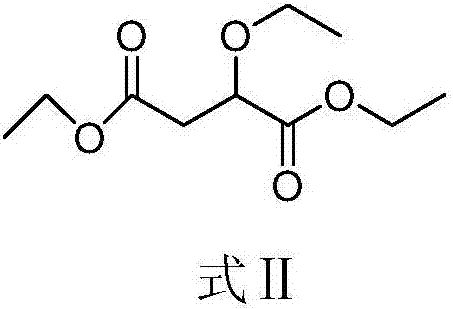
[0046] (1) Synthesis of compound shown in formula Ⅱ
[0047] Get 50g diethyl maleate and 50mL absolute ethanol in the 500mL reaction flask, add dropwise 20% (mass fraction) ethanol solution 98g of sodium ethylate at room temperature (the mol ratio of diethyl maleate and sodium ethylate is 1 : 1), the dropwise addition was completed in 5 minutes, followed by room temperature (20° C.) for 1 hour. The crude product of the compound represented by formula II can be obtained after the liquid phase detection of the raw material disappears completely.
[0048] According to the following NMR characterization data, the structure of the obtained compound is correct:
[0049] 1 H NMR (400MHz, CDCl 3 )δ4.33–4.11 (m, 5H), 3.73 (dq, J=14.1, 7.0Hz, 1H), 3.52 (dq, J=14.1, 7.0Hz, 1H), 2.74 (qd, J=15.9, 6.6Hz ,2H),1.34–1.17(m,9H).
[0050] (2) Synthesis of ...
Embodiment 2
[0063] Embodiment 2, the synthesis of trinexapac-ethyl
[0064] Carry out according to following reaction equation:
[0065]
[0066] (1) Synthesis of compound shown in formula Ⅱ
[0067] Get 50g diethyl maleate and 50mL absolute ethanol in the 500mL reaction flask, add dropwise 20% (mass fraction) ethanol solution 98g of sodium ethylate at room temperature (the mol ratio of diethyl maleate and sodium ethylate is 1 : 1), the dropwise addition was completed in 5 minutes, followed by room temperature (20° C.) for 1 hour. The crude product of the compound represented by formula II can be obtained after the liquid phase detection of the raw material disappears completely.
[0068] According to the following NMR characterization data, the structure of the obtained compound is correct:
[0069] 1 H NMR (400MHz, CDCl 3 )δ4.33–4.11 (m, 5H), 3.73 (dq, J=14.1, 7.0Hz, 1H), 3.52 (dq, J=14.1, 7.0Hz, 1H), 2.74 (qd, J=15.9, 6.6Hz ,2H),1.34–1.17(m,9H).
[0070] (2) Synthesis of comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com