Method for electrically converting aluminum chloride into aluminum oxide
A technology of aluminum chloride and alumina, applied in the field of electrolysis, can solve the problems of high energy consumption, high cost, large pollution, etc., and achieve the effects of high purity, short process and lower production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] In this embodiment, the method for electroconverting aluminum chloride into alumina is carried out in the following steps:
[0050] Step 1: electrolyze the aluminum chloride aqueous solution, the process parameters of the electrolysis are: the temperature is 20°C, and the voltage of the electrolysis is 3V;
[0051] In described step 1, the mass concentration of described aluminum chloride aqueous solution is 50g / L;
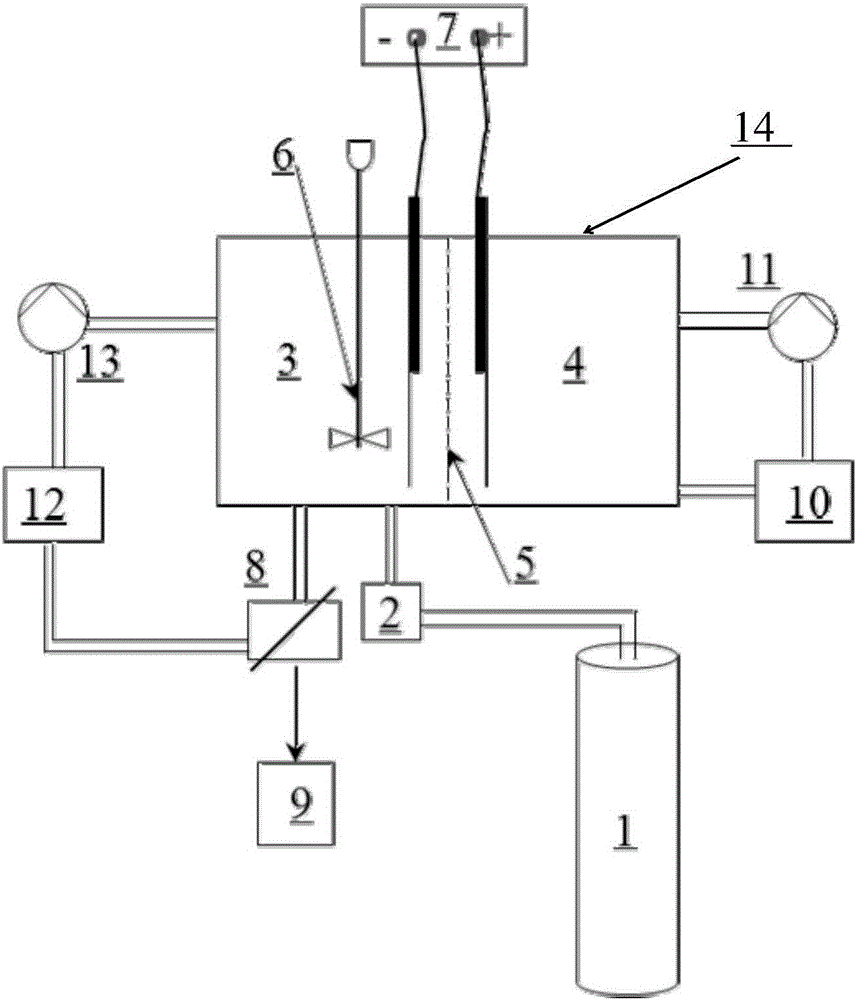
[0052] Step 2: Introduce high-purity carbon dioxide gas into the cathode chamber 3 electrolyte in the cationic membrane electrolyzer with a flow rate of 20m 3 / h, make the precipitation of hydrocarbon oxygen compounds that directly generate aluminum in the cathode chamber 3 of the cationic membrane electrolyzer;
[0053] In the step 2, the high-purity carbon dioxide gas is introduced through the vent hole at the bottom of the cathode chamber 3 of the electrolytic cell.
[0054] Step 3: Stir the cathode chamber 3 of the cationic membrane electrolyzer, the ...
Embodiment 2
[0061] In this embodiment, the method for electroconverting aluminum chloride into alumina is carried out in the following steps:
[0062] Step 1: electrolyzing the aluminum chloride aqueous solution, the process parameters of the electrolysis are as follows: the temperature is 90°C, and the voltage of the electrolysis is 20V;
[0063] In described step 1, the mass concentration of described aluminum chloride aqueous solution is 200g / L;
[0064] Step 2: Introduce high-purity carbon dioxide gas into the cathode chamber 3 electrolyte in the cationic membrane electrolyzer with a flow rate of 80m 3 / h, make the precipitation of hydrocarbon oxygen compounds that directly generate aluminum in the cathode chamber 3 of the cationic membrane electrolyzer;
[0065] In the step 2, the high-purity carbon dioxide gas is introduced through the vent hole at the bottom of the cathode chamber 3 of the electrolytic cell.
[0066] Step 3: Stir the cathode chamber 3 of the cationic membrane ele...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com