A kind of trifluoromethane resource utilization method
A technology of trifluoromethane and resource utilization, applied in the field of fluorine-containing olefins, can solve the problems of complex product components, loss of catalytic activity, melting, etc., and achieve the effect of improving process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] A resource utilization method of trifluoromethane, the mixture of trifluoromethane and nitrogen with a dilution ratio of 10% is used as a reaction raw material, and the reaction equation is:
[0058] CHF 3 → CF 2 = CF 2 +CF 3 CF=CF 2 +HF
[0059] Charge 180 g of trifluoromethane into a steel cylinder with a volume of 8 L, and then fill with 7 g of nitrogen gas for premixing.
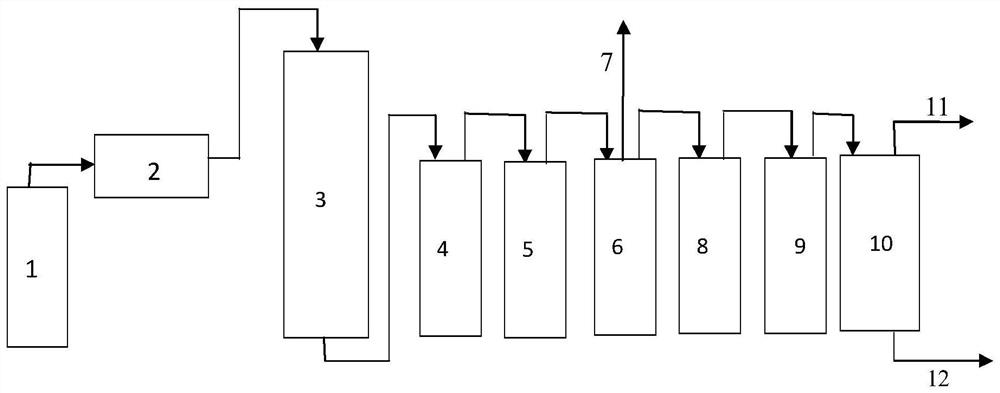
[0060] Place the SUS316 stainless steel preheating device with an inner diameter of 13 mm and a length of 95 cm and the SUS316 reaction device with an inner diameter of 13 mm and a length of 35 cm in the ceramic tube of the heating furnace. The ceramic tube is surrounded by heating elements and is insulated with aluminum silicate insulation cotton. The temperature required for the reaction is controlled by a heating furnace with a temperature controller and a thermocouple.
[0061] Adjust the readings of the temperature controller, first heat the preheating device and the reaction device, wh...
Embodiment 2
[0064] As described in Example 1, the difference is: adjust the real number of the mass flow meter, control the flow rate of the raw material to 1.4L / min, and control the system pressure to 0.4MPa. acid device, then dried, sampled, and gas chromatographically analyzed the content of each component in the mixed product.
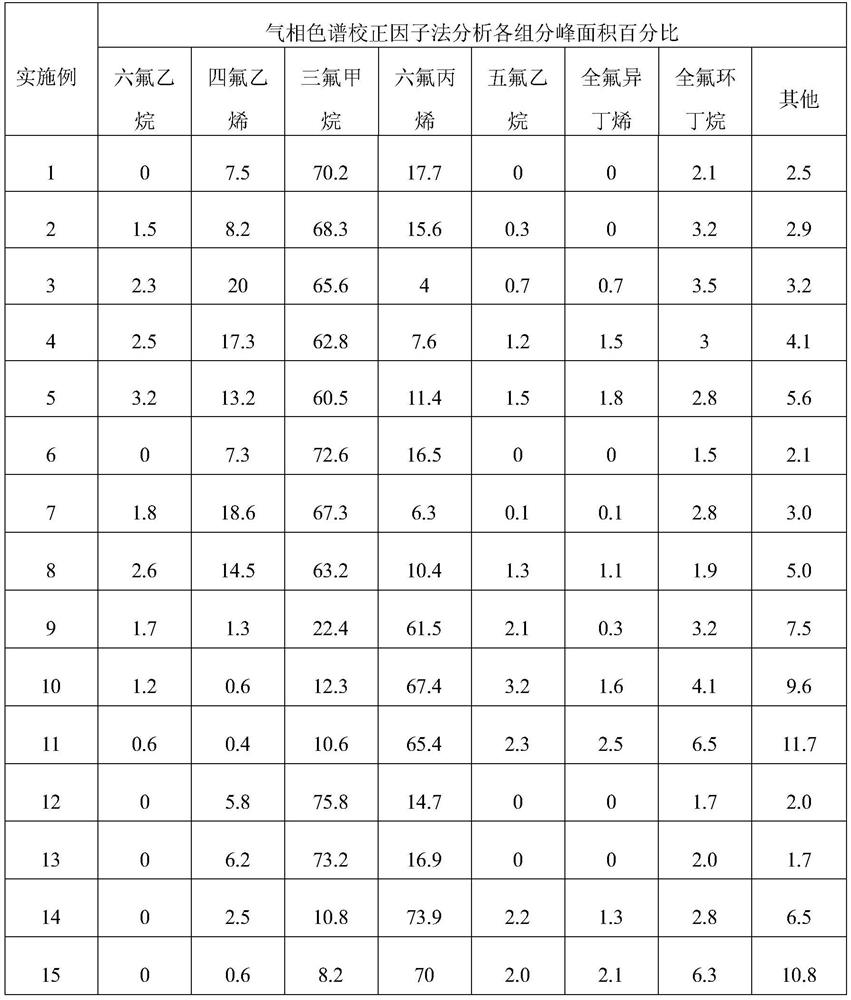
[0065] The product mixture after quenching alkali washing to remove acid enters the drying device, and the dried product mixture is passed into the methanol absorption device to remove the highly toxic perfluoroisobutene, and then enters the extraction and rectification separation device to obtain high-purity tetrafluoroethylene. , Hexafluoropropylene. The gas chromatography analysis results are listed in Table 1.
Embodiment 3
[0067] As described in Example 1, the difference is: adjust the reading of the temperature controller, control the reaction temperature of the reaction device to 750°C, adjust the real number of the mass flow meter, control the flow rate of the raw material to 0.5L / min, and control the system pressure to 0.4MPa. The product mixed flow from the reaction device is introduced into a quenching acid removal device filled with lye, then dried and sampled, and the content of each component in the mixed product is analyzed by gas chromatography.
[0068] The product mixture after quenching alkali washing to remove acid enters the drying device, and the dried product mixture is passed into the methanol absorption device to remove the highly toxic perfluoroisobutene, and then enters the extraction and rectification separation device to obtain high-purity tetrafluoroethylene. , Hexafluoropropylene. The gas chromatography analysis results are listed in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com