Mitochondria-targeted dihydropyridine derivative as well as preparation method and application thereof
A technology of dihydropyridine and mitochondria, applied in the field of medicine, can solve the problems of scavenging reactive oxygen radicals, NADPH structure aggregation, etc., and achieve the effects of easy product availability and simple preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
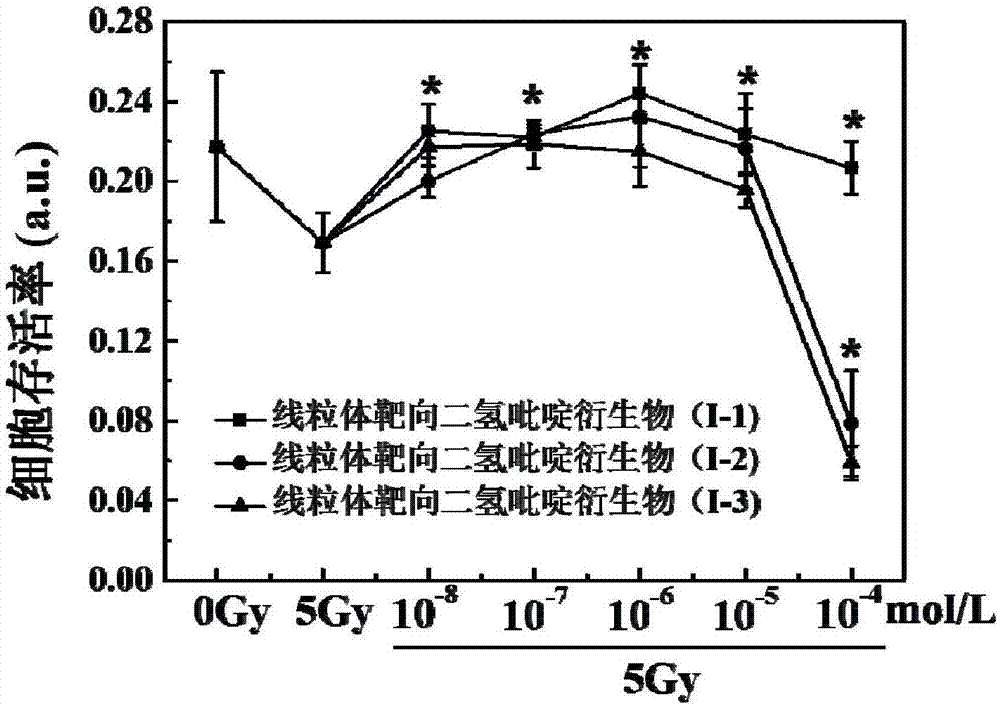
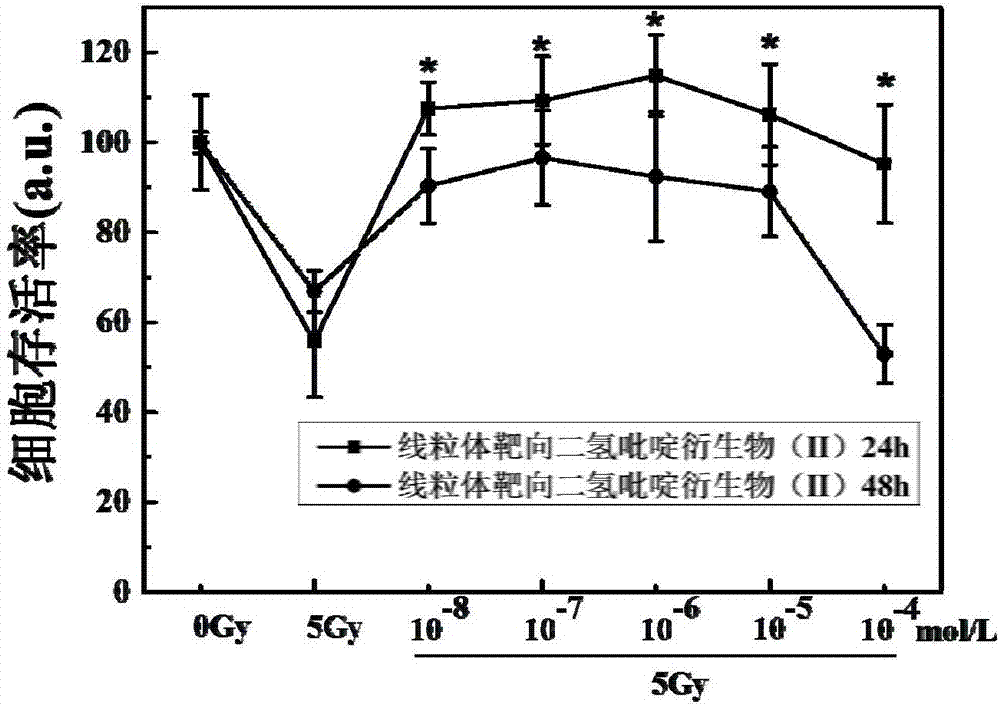
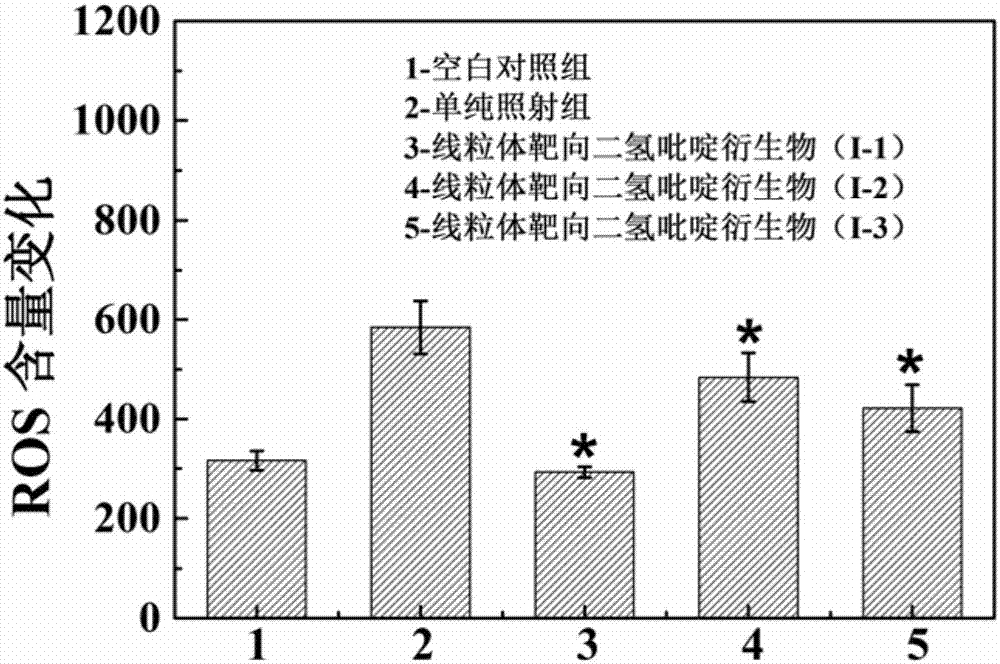
[0036] A preparation method for mitochondrial targeting dihydropyridine derivatives (I-1), comprising the steps of:
[0037] (1) Add triphenylphosphine (9.4g, 35.9mmol), 3-bromo-1 propanol (0.5g, 3.6mmol) and 10ml N,N-di Methylformamide (DMF), stirred and refluxed at 100°C for 10h, cooled to room temperature after the reaction, filtered the precipitated solid in the system under reduced pressure and washed the solid with cold DMF 3 times to obtain a white solid, dried overnight at 50°C to obtain a white Powdered solid (3-hydroxypropyl) triphenylphosphine bromide;
[0038] (2) Add NH to a 100ml three-necked flask equipped with magnetic stirring 4 HCO 3 (7.9g, 0.1mol), 40% aqueous formaldehyde solution (containing 0.1mol formaldehyde) and ethyl acetoacetate (26g, 0.2mol) and 60ml volume concentration of 50% ethanol aqueous solution, under the protection of nitrogen, heat and reflux for 20h until Pale yellow precipitate, the reaction system was cooled to room temperature, the ...
Embodiment 2
[0044] A method for preparing mitochondria-targeted dihydropyridine derivatives (I-2), comprising the steps of:
[0045] (1) (1) with embodiment 1;
[0046] (2) replace the formaldehyde in the embodiment (2) with the benzaldehyde of equimolar, other is with embodiment 2;
[0047] (3) Add compound 8 (where R is phenyl) (600mg, 2.0mmol), HOBT (162mg, 1.2mmol) and DMF (15mL) into a 100ml round bottom flask equipped with magnetic stirring and stir for half an hour, then add EDCI (230 mg, 1.2 mmol), (3-hydroxypropyl)triphenylphosphine bromide (514 mg, 1 mmol) obtained in step 1) and triethylamine (202 mg, 2 mmol). Under the protection of nitrogen, it was reacted overnight in the dark, and most of the DMF was evaporated under reduced pressure, and the saturated NaHCO 3 An aqueous solution (20 mL) was added to the reaction system, and a pale yellow solid was precipitated, which was collected by suction filtration under reduced pressure, dried and purified by column chromatography t...
Embodiment 3
[0052] A preparation method for mitochondrial targeting dihydropyridine derivatives (I-3), comprising the steps of:
[0053] (1) (1) with embodiment 1;
[0054] (2) replace the formaldehyde in the embodiment (2) with the thiophene 2-formaldehyde of equimolar, other is with embodiment 2;
[0055] (3) Add compound 8 (where R is thienyl) (307mg, 1.0mmol), HOBT (162mg, 1.2mmol) and DMF (15mL) into a 100ml round bottom flask equipped with magnetic stirring and stir for half an hour, then add EDCI (230 mg, 1.2 mmol), (3-hydroxypropyl)triphenylphosphine bromide (514 mg, 1 mmol) obtained in step 1) and triethylamine (202 mg, 2 mmol). Under the protection of nitrogen, it was reacted overnight in the dark, and most of the DMF was evaporated under reduced pressure, and the saturated NaHCO 3 An aqueous solution (20 mL) was added to the reaction system, and a pale yellow solid was precipitated, which was collected by suction filtration under reduced pressure, dried and purified by column...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com