Complex catalyst, catalyst composition and preparation method of a kind of olefin polymer
A technology of catalyst and composition, which is applied in the field of preparation of compounds, complex catalysts, catalyst compositions and olefin polymers, and can solve the problems of poor adhesion, wettability and miscibility, metal deactivation, and the inability to achieve polar monolithic Copolymerization and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0079] The present invention has no special limitation on the preparation method of the compound represented by the structure of formula (I), just use the conventional preparation method well known to those skilled in the art, and those skilled in the art can according to the actual production situation, product requirements and use requirements To select and adjust, the present invention is to optimize the preparation process, a complete technical scheme, the preparation method of the compound shown in the formula (I) structure is specifically preferably the following steps:
[0080] (a) by reacting the diketone of formula A and the amine compound of formula B, form the compound of formula C; As shown in reaction formula (a):
[0081]
[0082] (b) react by formula C compound and formula D amine compound, form formula (I) compound; As shown in reaction formula (b):
[0083]
[0084] In the preparation method of the above-mentioned compound represented by the structure of...
Embodiment 1
[0131] Synthesis of Ligand NO-L
[0132]
[0133] 2,6-Dimorpholine aniline (1.05 g, 4 mmol) was added to a 20 mL Schlenk bottle under nitrogen (Irie, Y.; Koga, Y.; Matsumoto, T.; Matsubara, K. Eur. J .Org.Chem.2009, 2243.), 2,3-butanedione (0.174 ml, 2 mmol), formic acid (0.05 ml, 1.33 mmol) and methanol (6 ml). After stirring at room temperature for three days, a yellow solid precipitated. After filtration, the solid precipitate was washed three times with ice methanol and then dried by vacuum pump to obtain the target product NO-L (0.737 g, yield 64%).
[0134] The product prepared in Example 1 of the present invention is detected by proton nuclear magnetic spectrum and carbon spectrum, and the results are as follows:
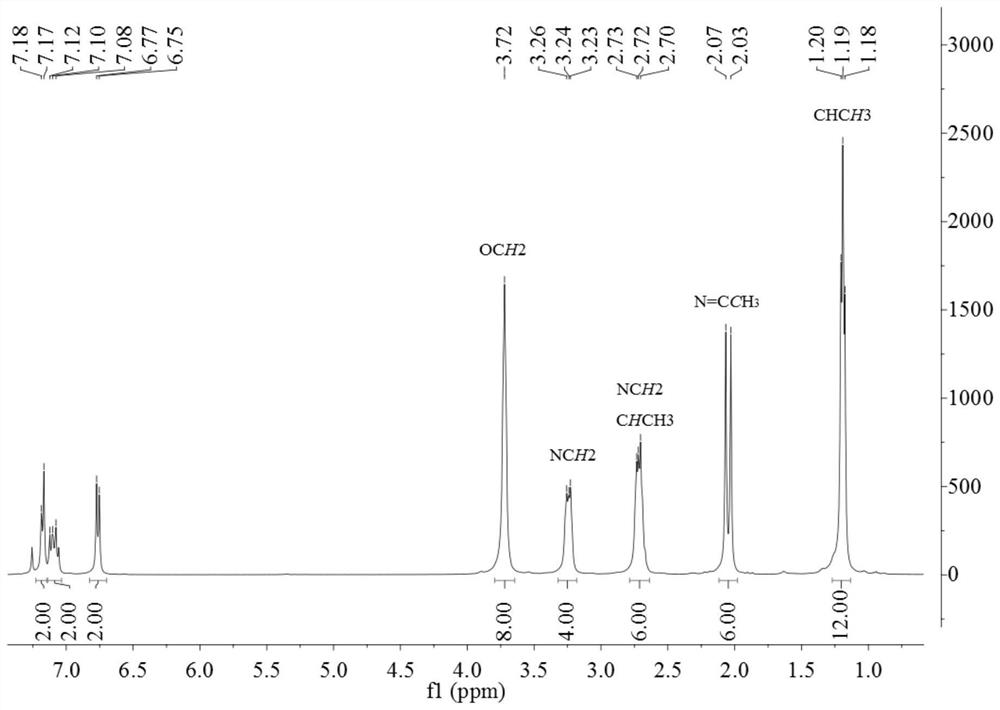
[0135] 1 H NMR (400MHz, CDCl 3 )δ7.06(t, J=8.0Hz, 2H, ArH), 6.74(d, J=8.1Hz, 4H, ArH), 3.88–3.65(m, 16H, OCH 2 ),3.39–3.17(m,8H,NCH 2 ),2.80–2.60(m,8H,NCH 2 ), 1.93(s,6H,N=CCH 3 ).
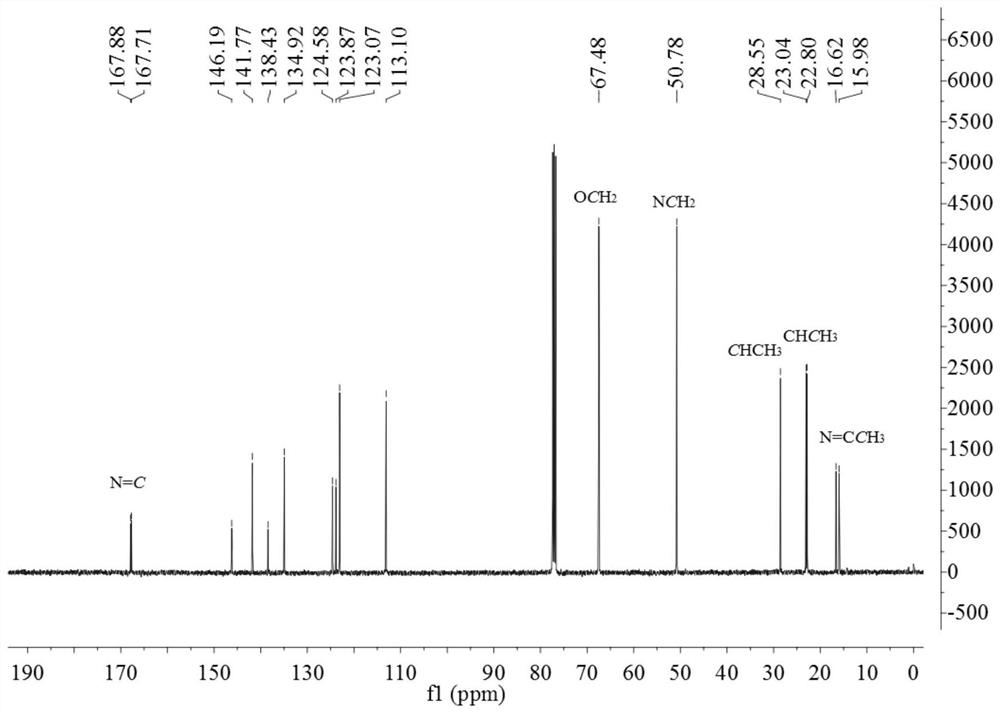
[0136] 13 C NMR (100MHz, CDCl 3 )δ166.73 (N=C), 141.94, 138.62...
Embodiment 2
[0140] Synthesis of Ligand N6-L
[0141]
[0142] 2,6-Dihexahydropyridineaniline (1.04 g, 4 mmol) was added to a 20 mL Schlenk bottle under nitrogen (Irie, Y.; Koga, Y.; Matsumoto, T.; Matsubara, K. Eur. J. Org. Chem. 2009, 2243.), 2,3-butanedione (0.174 mL, 2 mmol), formic acid (0.05 mL, 1.33 mmol) and methanol (6 mL). After stirring at room temperature for three days, a yellow solid precipitated. After filtration, the solid precipitate was washed three times with ice methanol and then dried by vacuum pump to obtain the target product N6-L (0.795 g, yield 70%).
[0143] The product prepared in Example 2 of the present invention is detected by proton nuclear magnetic spectrum and carbon spectrum, and the results are as follows:
[0144] 1 H NMR (400MHz, CDCl 3 )δ6.97(t, J=8.0Hz, 2H, ArH), 6.66(d, J=8.0Hz, 4H, ArH), 3.22(d, J=7.5Hz, 8H, NCH 2 ),2.69–2.56(m,8H,NCH 2 ), 1.95(s,6H,N=CCH 3 ), 1.69–1.61 (m, 8H, NCH 2 CH 2 CH 2 ),1.57–1.47(m,16H,NCH 2 CH 2 ).
[0145]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| Branching factor | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com