Medicine for treating chronic gastritis and hyperacidity and preparation method thereof
A technology for hyperacidity and chronic gastritis, applied in the field of pharmaceuticals, can solve the problems of slow patient symptoms, disintegration and slow onset, and low bioavailability, so as to improve bioavailability and reduce adverse effects. Response, high bioavailability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] A kind of preparation method that the medicine for treating chronic gastritis, hyperacidity is prepared into dispersible tablet, comprises the steps:
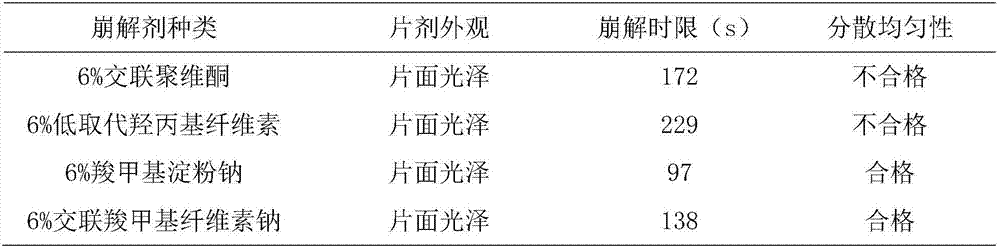
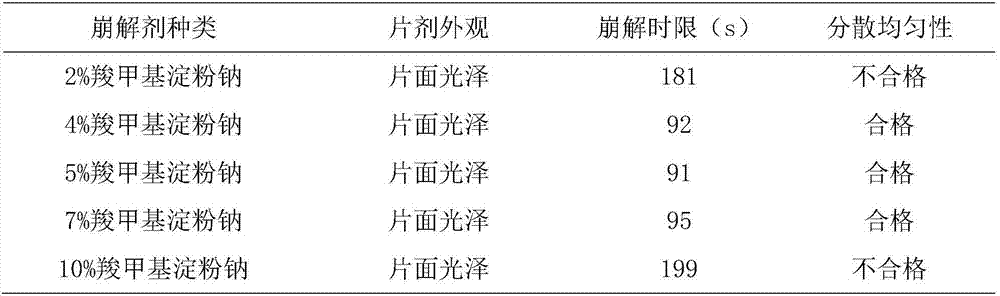
[0065] T1: Weigh the following components by weight: 2000g sucralfate, 400g crospovidone, 200g hydroxypropyl methylcellulose, 1200g dextran, 200g sodium carboxymethyl starch, sodium lauryl sulfate 200g, micropowder silica gel 100g, magnesium stearate 100g, pass through a 100-mesh sieve, set aside;
[0066] T2: Preparation of sucralfate solid dispersion: take crospovidone and put it in the batching pot, add appropriate amount of absolute ethanol and heat at 35°C to dissolve, keep the temperature at 35°C and add sucralfate, keep stirring for 5 hours , placed at room temperature, dried at 50°C for 10 h under a vacuum of -0.06Mpa, taken out, pulverized, and passed through an 80-mesh sieve to obtain a sucralfate solid dispersion, whose moisture content was determined to be 5.8%;
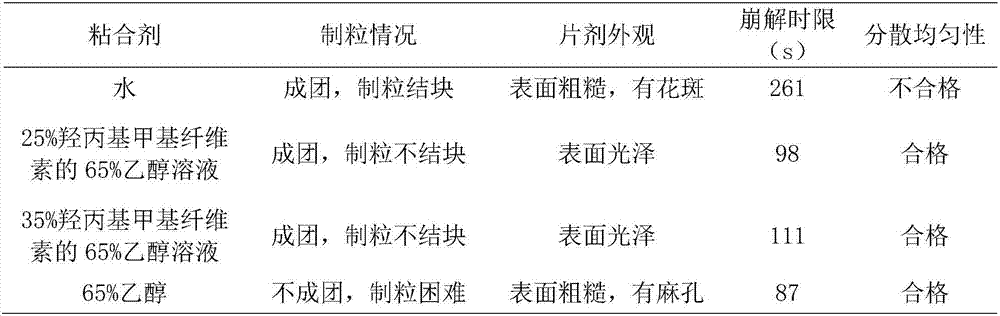
[0067] T3: Dissolve hydroxypropyl methylcell...
Embodiment 2
[0073] A kind of preparation method that the medicine for treating chronic gastritis, hyperacidity is prepared into dispersible tablet, comprises the steps:
[0074] T1: Weigh the following components by weight: 2000g sucralfate, 800g crospovidone, 400g hydroxypropyl methylcellulose, 1600g dextran, 400g sodium carboxymethyl starch, sodium lauryl sulfate 400g, micropowder silica gel 140g, magnesium stearate 140g, pass through a 100-mesh sieve, set aside;
[0075] T2: Preparation of sucralfate solid dispersion: take crospovidone and put it in the batching pot, add appropriate amount of absolute ethanol and heat at 45°C to dissolve, keep the temperature at 45°C and add sucralfate, keep stirring for 4 hours , placed at room temperature, dried at 65°C for 8 hours under a vacuum of -0.08Mpa, taken out, pulverized, and passed through an 80-mesh sieve to obtain a sucralfate solid dispersion, and its moisture content was determined to be 4.5%;
[0076] T3: take hydroxypropyl methylcel...
Embodiment 3
[0082] A kind of preparation method that the medicine for treating chronic gastritis, hyperacidity is prepared into dispersible tablet, comprises the steps:
[0083] T1: Weigh the following components by weight: 2000g sucralfate, 600g crospovidone, 300g hydroxypropyl methylcellulose, 1400g dextran, 300g sodium carboxymethyl starch, sodium lauryl sulfate 300g, 120g of micropowder silica gel, 120g of magnesium stearate, pass through a 100-mesh sieve, and set aside;
[0084] T2: Preparation of sucralfate solid dispersion: take crospovidone and put it in the batching pot, add appropriate amount of absolute ethanol and heat at 40°C to dissolve, keep the temperature at 40°C and add sucralfate, keep stirring for 4.5 hours , placed at room temperature, dried at 60°C for 9 hours under a vacuum of -0.09Mpa, taken out, pulverized, and passed through an 80-mesh sieve to obtain a sucralfate solid dispersion, whose moisture content was determined to be 3.2%;
[0085] T3: take hydroxypropyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com