Imidaclothiz synthesis method
A synthesis method and chlorothiline technology, applied in the direction of organic chemistry and the like, can solve the problems of decreased product content and yield, decreased product content, difficult removal of double-substituted by-products, etc., and achieve the effect of improving yield and content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
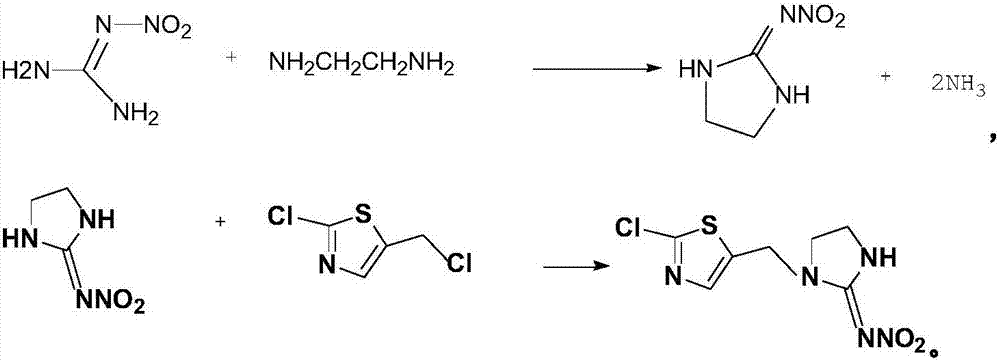
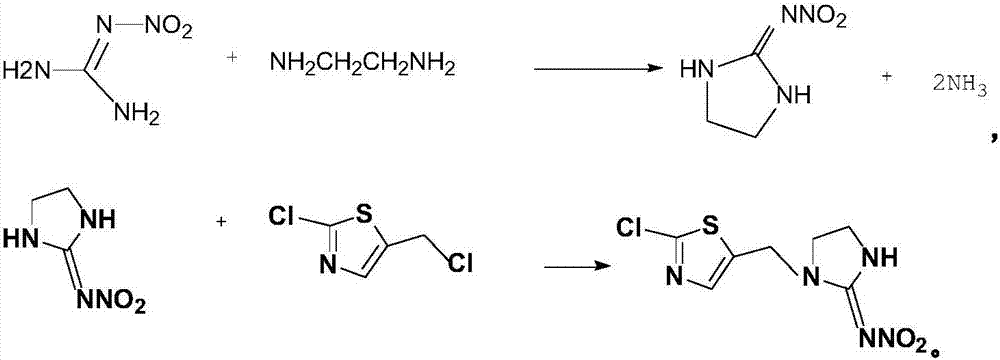
[0016] The synthetic method of present embodiment chlorothialine, this synthetic method adopts nitroguanidine, ethylenediamine to react, and the intermediate of generation prepares chlorothialine with 2-chloro-5-chloromethylthiazole, concrete steps are as follows:
[0017] Add 125g of nitroguanidine and 50g of 70% sulfuric acid to a 500ml four-necked flask in sequence, add 32g of ethylenediamine dropwise, heat up to 80°C for reaction, and filter, wash and dry to obtain the intermediate imidazolidine after the reaction is completed.
[0018] Add 100mL of toluene, 132g of imidazolidine, 20g of potassium carbonate, and tetrabutylammonium bromide into a 1000ml four-neck flask, mix a little, add dropwise 200mL of toluene solution in which 2-chloro-5-chloromethylthiazole is dissolved, and heat at 45°C After reacting for 6 hours, the temperature was lowered, the layers were separated, and 247 g of a yellow solid was obtained by precipitation.
[0019] The content of chlorothialine in...
Embodiment 2
[0021] The synthetic method of present embodiment chlorothialine, this synthetic method adopts nitroguanidine, ethylenediamine to react, and the intermediate of generation prepares chlorothialine with 2-chloro-5-chloromethylthiazole, concrete steps are as follows:
[0022] Add 125g of nitroguanidine, 50g of 70% sulfuric acid in sequence in a 500ml four-neck flask, dropwise add 32g of ethylenediamine, raise the temperature to 90°C for reaction, and filter, wash and dry the intermediate product imidazolidine after the reaction is completed.
[0023] Add 100mL of toluene, 132g of imidazolidine, 20g of potassium carbonate, and tetrabutylammonium bromide into a 1000ml four-neck flask, mix a little, add dropwise 200mL of toluene solution in which 2-chloro-5-chloromethylthiazole is dissolved, and heat at 49°C After reacting for 8 hours, the temperature was lowered, the layers were separated, and 249 g of a yellow solid was obtained by precipitation.
[0024] The content of chlorothia...
Embodiment 3
[0026] The synthetic method of present embodiment chlorothialine, this synthetic method adopts nitroguanidine, ethylenediamine to react, and the intermediate of generation prepares chlorothialine with 2-chloro-5-chloromethylthiazole, concrete steps are as follows:
[0027] Add 125g of nitroguanidine and 50g of 70% sulfuric acid successively into a 500ml four-neck flask, dropwise add 32g of ethylenediamine, raise the temperature to 85°C for reaction, and filter, wash and dry to obtain the intermediate imidazolidine after the reaction is completed.
[0028] Add 100mL of toluene, 132g of imidazolidine, 20g of potassium carbonate, and tetrabutylammonium bromide into a 1000ml four-neck flask, mix a little, add dropwise 200mL of toluene solution dissolved in 2-chloro-5-chloromethylthiazole, and heat at 47°C After reacting for 7 hours, the temperature was lowered, the layers were separated, and 252 g of a yellow solid was obtained by precipitation.
[0029] In the present embodiment,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com