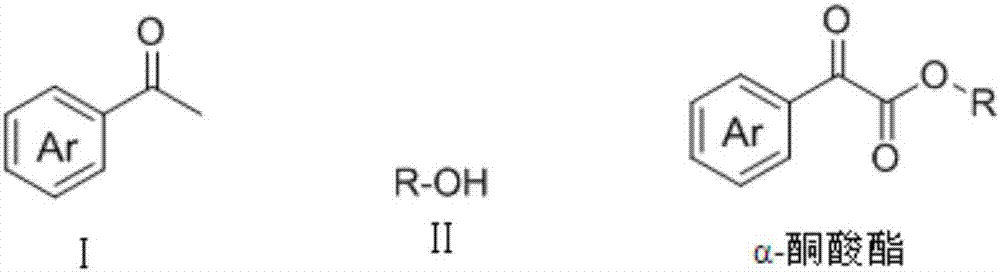
Method for preparing alpha-keto ester by microfluidic chip reactor
A microfluidic chip and reactor technology, applied in chemical instruments and methods, formation/introduction of carboxylate groups, preparation of carboxylate esters, etc., can solve the problems of long reaction process cycle, harsh reaction conditions, and poor economy, etc. Achieve the effect of good industrial scale-up potential, mild reaction conditions and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Synthesis of compound 3a:
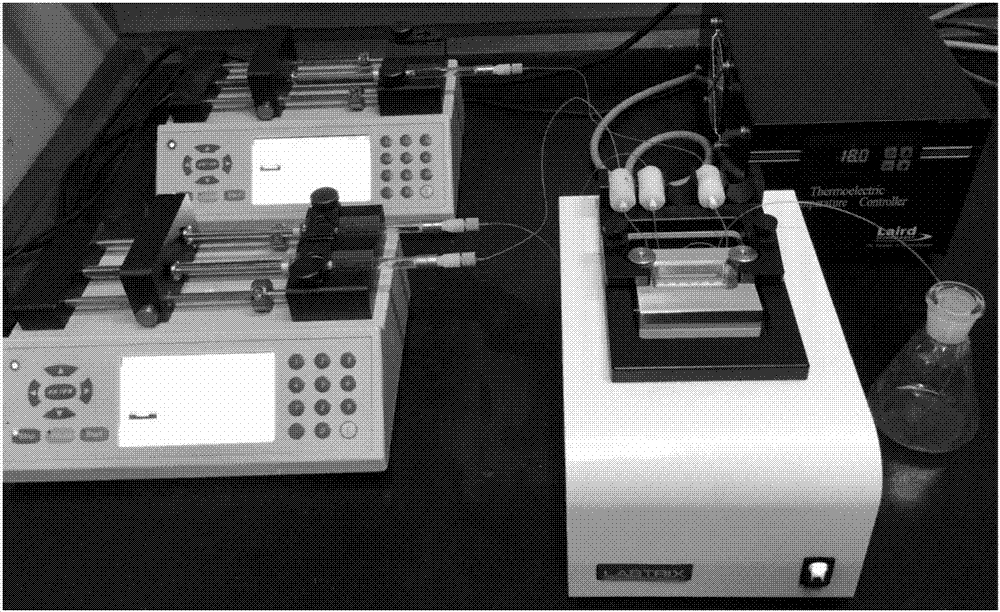
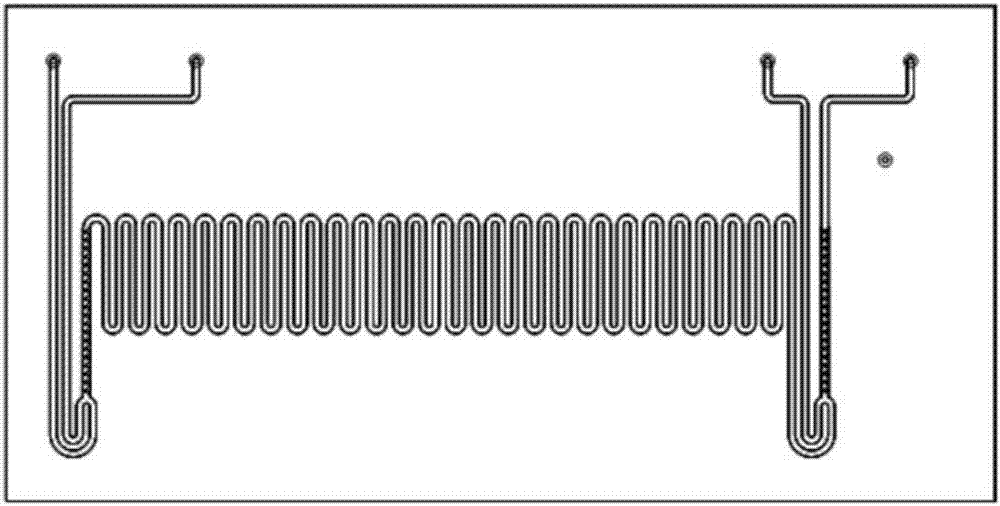
[0035] Dissolve 2mmol (0.24g) of acetophenone, 10mmol (1.22g) of phenylethyl alcohol, 0.6mmol (0.15g) of iodine, and 2mmol (0.30g) of 1,8-diazabicyclo[5.4.0]undecene (DBU). In 3mL N,N-dimethylformamide (DMF), a homogeneous solution A was obtained, which was added to the microsyringe pump a; 8mmol (1.02g) of 70% tert-butanol hydroperoxide solution was dissolved in 3 mLN, In N-dimethylformamide (DMF), a homogeneous solution B was obtained, which was added to microsyringe pump b; saturated sodium thiosulfate was added to microsyringe pump c; microsyringe pumps a, b, c The injection flow rate was 5 μl / min, the microfluidic chip reaction volume V=10 μl (reaction time 1min), and the reactor temperature was 130°C; after two cycles of reaction (2min), the reaction liquid was collected, and the product yield was calculated by HPLC. The yield was 84%, and the product 3a was obtained after separation by column chromatography; 1 H NMR (400MHz, CDCl 3 ...
Embodiment 2
[0037]Synthesis of compound 3b:
[0038] 2mmol (0.31g) p-chloroacetophenone, 10mmol (1.22g) phenylethyl alcohol, 0.6mmol (0.15g) iodine, 2mmol (0.30g) 1,8-diazabicyclo [5.4.0] undecene (DBU ) was dissolved in 3mL N,N-dimethylformamide (DMF) to obtain a homogeneous solution A, which was added to the microsyringe pump a; 8mmol (1.02g) 70% tert-butanol hydroperoxide solution was dissolved in 3mLN , in N-dimethylformamide (DMF), to obtain a homogeneous solution B, which was added to microinjection pump b; saturated sodium thiosulfate was added to microinjection pump c; microinjection pumps a, b, c The injection flow rate is 5 μl / min, the microfluidic chip reaction volume V=10μl (reaction time 1min), and the reactor temperature is 130°C; after two cycles of reaction (2min), the reaction liquid is collected and calculated by HPLC The product yield was 88%, and the product 3b was obtained after separation by column chromatography; 1 H NMR (400MHz, CDCl3) δ7.80 (dd, J = 8.9, 5.4Hz, ...
Embodiment 3
[0040] Synthesis of compound 3c:
[0041] 2mmol (0.33g) m-nitroacetophenone, 10mmol (1.22g) phenethyl alcohol, 0.6mmol (0.15g) iodine, 2mmol (0.30g) 1,8-diazabicyclo [5.4.0] undecene ( DBU) was dissolved in 3 mL N,N-dimethylformamide (DMF) to obtain a homogeneous solution A, which was added to the microsyringe pump a; 8 mmol (1.02 g) of 70% tert-butanol hydroperoxide solution was dissolved in In 3mL N,N-dimethylformamide (DMF), obtain a homogeneous solution B, which is added to microsyringe pump b; saturated sodium thiosulfate is added to microsyringe pump c; microsyringe pumps a, b, The injection flow rate of c is 5 μl / min, the microfluidic chip reaction volume V=10μl (reaction time 1min), and the reactor temperature is 130°C; after two cycles of reaction (2min), the reaction liquid is collected and analyzed by HPLC The calculated product yield was 86%, and the product 3c was obtained after column chromatography separation; 1 H NMR (400MHz, CDCl 3 )δ8.86–8.64(m,1H),8.33(dd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com