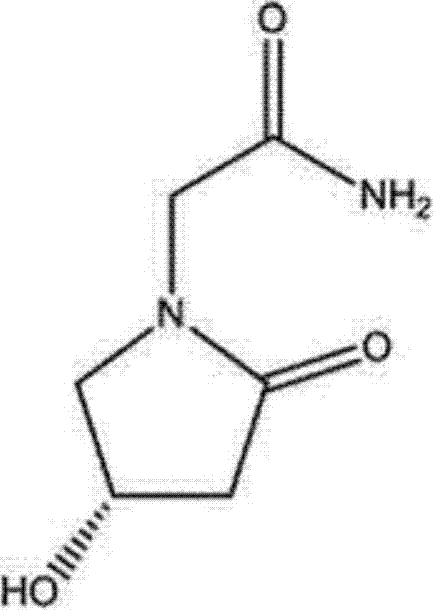
Levorotatory oxiracetam freeze-drying powder injection and preparation method thereof
A freeze-dried powder injection and freeze-drying technology, used in freeze-dried delivery, powder delivery, pharmaceutical formulations, etc., can solve the problems of inconsistent properties of the upper and lower layers, difficult to form a skeleton, and poor product stability, and achieve consistent properties of the upper and lower layers. Good compliance and good product stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] A levoxiracetam freeze-dried powder injection, prepared according to the following steps:
[0022] Element
[0023] Makes 1000 bottles
[0024] Preparation process:
[0025] 1. Concentrated preparation: put the raw and auxiliary materials of the prescribed amount in a container, add 5 times the weight of levoxiracetam in sterile water for injection and stir, after dissolving, add activated carbon for needles with a mass fraction of 0.5%, stir for 30 minutes, and then Filter with a 0.45 micron microporous membrane, collect the filtrate, and set aside;
[0026] 2. Dilute preparation: add sterilized water for injection to the filtrate to 1000 times the volume of the filtrate, adjust the pH to 7.0 with hydrochloric acid or sodium hydroxide, then sterilize and filter with a 0.22 micron microporous membrane, take the filtrate and fill it Packed in sterile glass bottles for later use;
[0027] 3. Freeze-drying: quickly freeze the temperature of the heat transfer o...
Embodiment 2
[0073] A levoxiracetam freeze-dried powder injection, prepared according to the following steps:
[0074] Element
Dosage (% by weight)
Levoxiracetam
40%
L-serine
25%
20%
sodium glutamate
8%
6%
1%
[0075] Makes 1000 bottles
[0076] Preparation process: prepared according to the preparation process of Example 1.
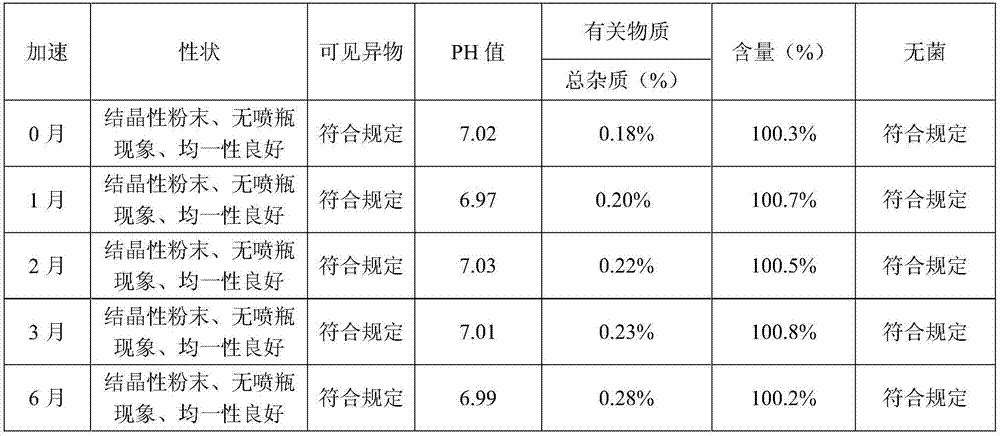
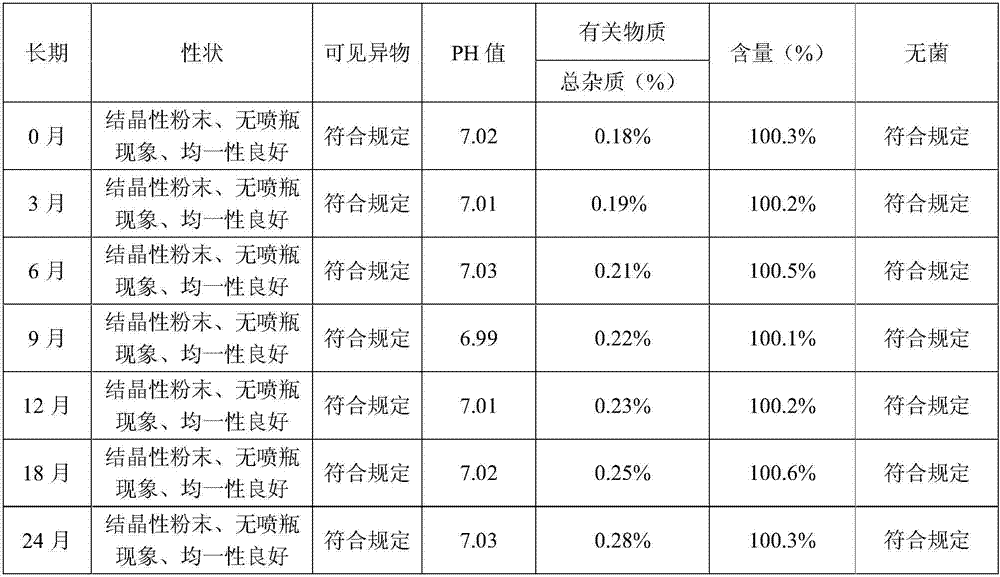
[0077] By the test method of embodiment 1, the result of the sample stability test in embodiment 2 shows that the sample quality is stable in accelerating June, and the quality is stable in 24 months for a long time, so this product is valid for at least 24 months; Squirt bottle phenomenon occurs. Example 2 The influence of the preparation process on the increase of impurities The experimental results show that the increase of product impurities in the preparation process of this product is small, which meets the product requirements. The results of the...
Embodiment 3
[0079] A levoxiracetam freeze-dried powder injection, prepared according to the following steps:
[0080] Element
Dosage (% by weight)
Levoxiracetam
38%
L-serine
26%
22%
8%
5%
1%
[0081] Makes 1000 bottles
[0082] Preparation process: prepared according to the preparation process of Example 1.
[0083] By the test method of embodiment 1, the sample stability test result of embodiment 3 shows that accelerated June sample quality is stable, and long-term 24 months are stable in quality, so this product is valid for at least 24 months; Squirt bottle phenomenon occurs. Example 3 The influence of the preparation process on the increase of impurities The experimental results show that the increase of product impurities in the preparation process of this product is small, which meets the product requirements. The results of the mouse writhing method t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com