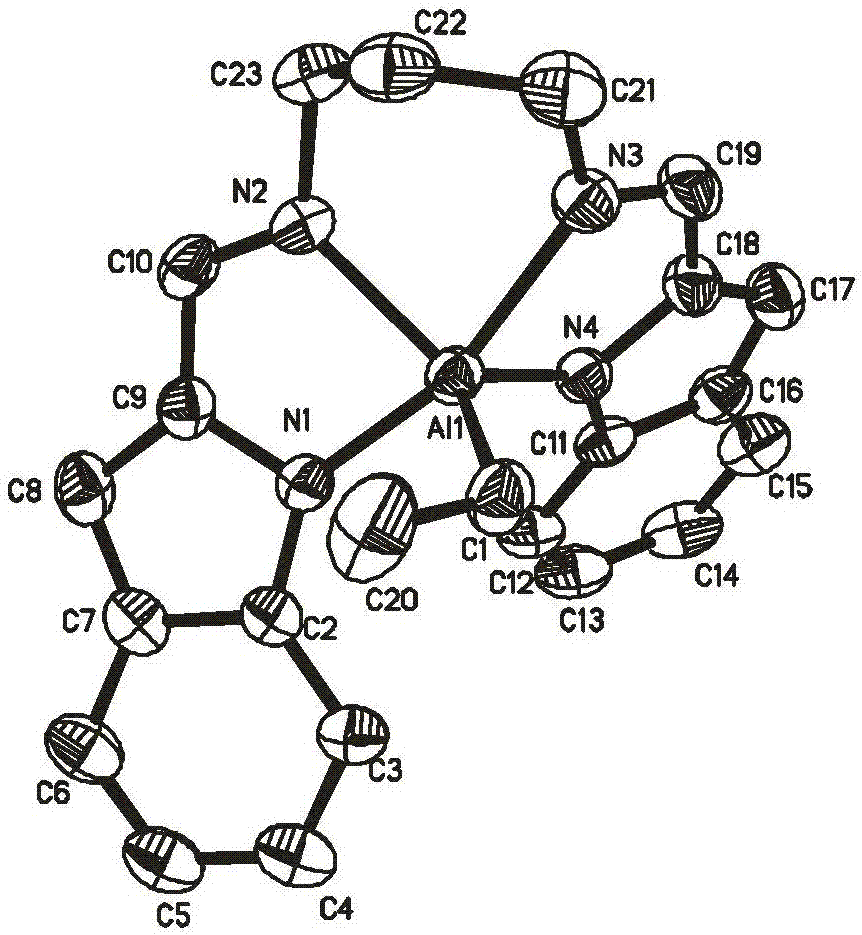
Aluminium alkyl coordination complexes containing N,N,N,N-tetradentate chelate pentacoordinate ligands, preparation method of aluminium alkyl coordination complexes and method for catalyzing ring opening polymerization of lactide
A technology of alkylaluminum and complexes, which is applied in the field of catalytic lactide ring-opening polymerization, can solve the problems of no activity and low activity, and achieve the effects of simple operation, low cost and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] In the preparation method provided by the present invention, the specific type of solvent can be selected within a wide range, but from the viewpoint of the cost of preparation, preferably, the solvent is selected from at least one of tetrahydrofuran, dichloromethane and toluene, preferably tetrahydrofuran .
[0032] In addition, in the above preparation method, the order of addition of the materials can be selected within a wide range, but considering the yield of the target product, preferably, in the preparation method, the order of addition of the materials is: The bisindole ligand is mixed with a solvent to form a ligand solution; then in the presence of a protective gas, the AlR 3 The solution is added dropwise to the ligand solution;
[0033] After the above-mentioned coordination reaction is completed, the purification method of the product can be selected in a wide range, but in order to simplify the purification steps and increase the purification yield, preferably...
preparation example 1
[0046] Preparation:
[0047] Dissolve indole 2-carboxaldehyde (30.0 mmol), 1,3-propanediamine (15.0 mmol), and p-toluenesulfonic acid (0.03 mmol) in 10 mL ethanol, react at 25°C for 12 hours, filter with suction, and wash with a small amount of cold ethanol Three times, vacuum drying, to obtain the bisimine bridged bisindole ligand with the structure shown in formula (B1).
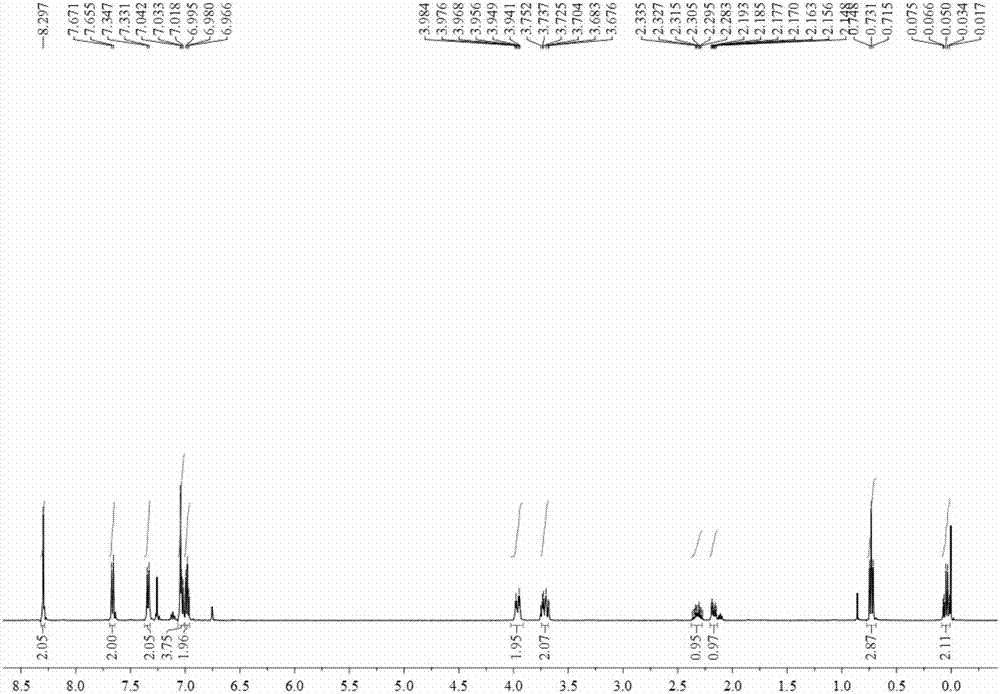
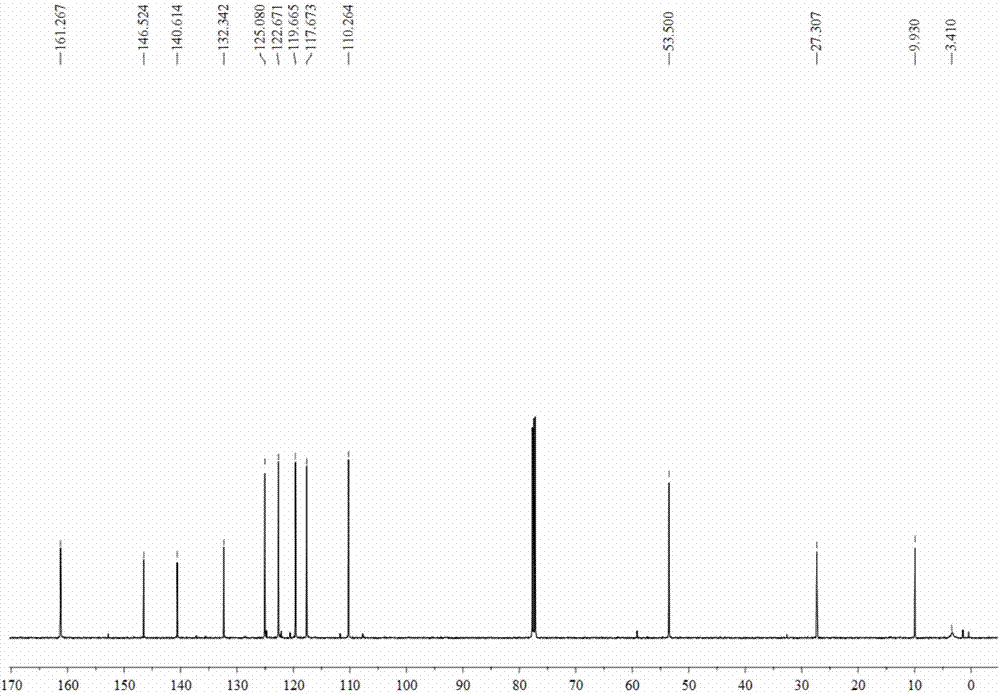
[0048] The characterization data of the product are: 1 H NMR (500MHz, CDCl3): δ9.68 (s, 2H), 8.26 (s, 2H), 7.64 (d, J = 7.5 Hz, 2H), 7.30 (d, J = 8.0 Hz, 2H), 7.24 ( t,J=8.0Hz,,2H), 7.11(t,J=7.5Hz,2H), 6.75(s,2H), 3.76-3.74(m,4H), 2.10-2.05(m,2H); 13 C NMR (75MHz, CDCl3): δ153.2, 137.3, 135.0, 128.0, 124.4, 121.8, 120.1, 111.6, 108.1, 58.4, 32.4; HRMS (APCI) m / z: calcd for C 21 H 21 N 4 (M+H + ) 329.1761; Found: 329.1764.
preparation example 2
[0050] Preparation:
[0051] The bisimine bisindole ligand with the structure shown in formula (B2) was prepared according to the method of Preparation Example 1, except that 1,3-propanediamine was replaced with ethylenediamine.
[0052] The characterization data of the product are: 1 H NMR(500MHz, CDCl 3 ): δ9.54(s, 2H), 8.23(s, 2H), 7.62(d,J=8.0Hz,2H), 7.33(d,J=8.0Hz,2H), 7.24(t,J=8.0Hz ,,2H), 7.09(t,J=7.5Hz,2H), 6.74(s,2H),3.92(s,3H); 13 C NMR(75MHz, CDCl 3 ):δ 154.2,137.3,135.1,128.0,124.4,121.8,120.1,111.6,108.2,60.9; HRMS(APCI) m / z:calcd for C 20 H 19 N 4 (M+H + ) 315.1604; Found: 315.1601.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com