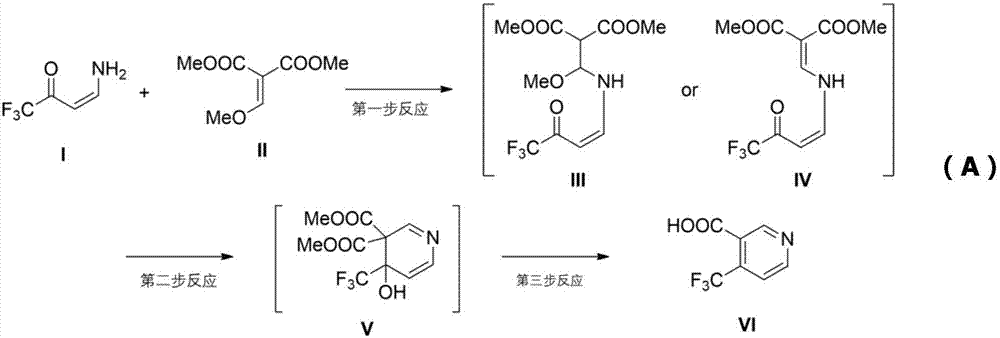
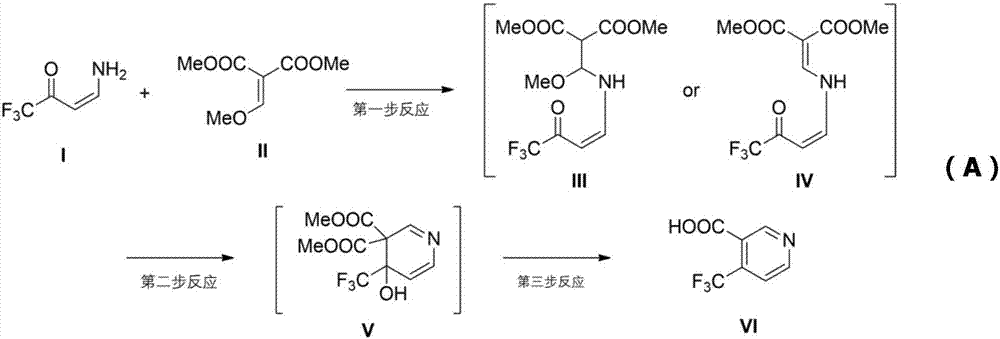
Method for synthesizing 4-trifluoromethyl nicotinic acid
A technology of trifluoromethyl nicotinic acid and dimethyl methoxymethylene malonate, which is applied in the direction of organic chemistry, can solve the problems of impossible industrialization, and achieve convenient post-processing, high feasibility, and equipment requirements low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] In a 500ml flask, add 1-methylpyrrolidone 100mL, sodium hydride 2.9g, stir and cool down to 0°C, add dropwise 1,1,1-trifluoro-4-aminobutenone 10g, control the temperature at 10°C Next, after reacting for 1 hour, 12.5 g of dimethyl 2-methoxymethylenemalonate was added, and reacted for 4 hours. Then add 200 mL of methanol, heat to 65°C, react for 3 hours, add 300 grams of concentrated hydrochloric acid (30% wt), heat to boiling, reflux for 9 hours, distill methanol off, add 300 grams of ice water, filter, and use the filter cake Washed with water, dried to obtain 6.8g yellow solid, yield 49%, the nuclear magnetic data of yellow solid is 1 HNMR (400MHz, DMSO-d6): d7.86(d, J=5.2Hz, 1H), 8.97(d, J=5.2Hz, 1H), 9.06(s, 1H), 14.04(bs, 1H), according to NMR data determined that the yellow solid was 4-trifluoromethylnicotinic acid, and the purity of 4-trifluoromethylnicotinic acid in the yellow solid was analyzed to be 98.7%.
Embodiment 2
[0033]In a 500ml flask, add DMF120mL, potassium tert-butoxide 12.4g, stir and cool down to 0°C, add 12.8g of 1,1,1-trifluoro-4-aminobutenone dropwise, control the temperature below 10°C, and react After 1 hour, 16.8 g of dimethyl 2-methoxymethylenemalonate was added and reacted for 3-5 hours. Then add 250 mL of ethanol, heat to 80°C, react for 2-3 hours, add 300 grams of concentrated hydrochloric acid, heat to boil, reflux for 8-10 hours, distill off ethanol, add 350 grams of ice water, filter, and wash the filter cake with water. Dry, obtain 6.2g yellow solid, productive rate 35%, the NMR data of yellow solid is 1 HNMR (400MHz, DMSO-d6): d7.86(d, J=5.2Hz, 1H), 8.97(d, J=5.2Hz, 1H), 9.06(s, 1H), 14.04(bs, 1H), according to NMR data determined that the yellow solid was 4-trifluoromethylnicotinic acid, and the purity of 4-trifluoromethylnicotinic acid in the yellow solid was analyzed to be 99.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com