Alkylthiol functionalized aromatic carboxylic acid compound and preparation method thereof
A technology of aromatic carboxylic acid and alkyl thiol, which is applied in the field of alkyl thiol functionalized aromatic carboxylic acid compound and its preparation, which can solve the problems of difficult compound modification and limited wide application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
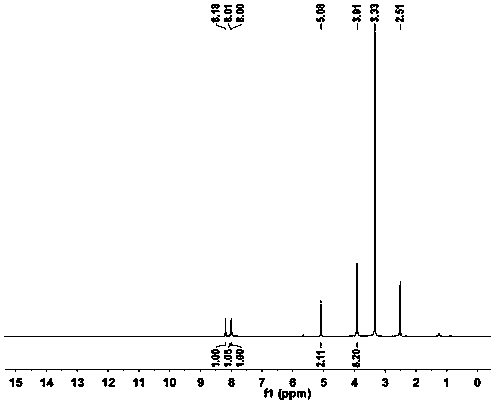
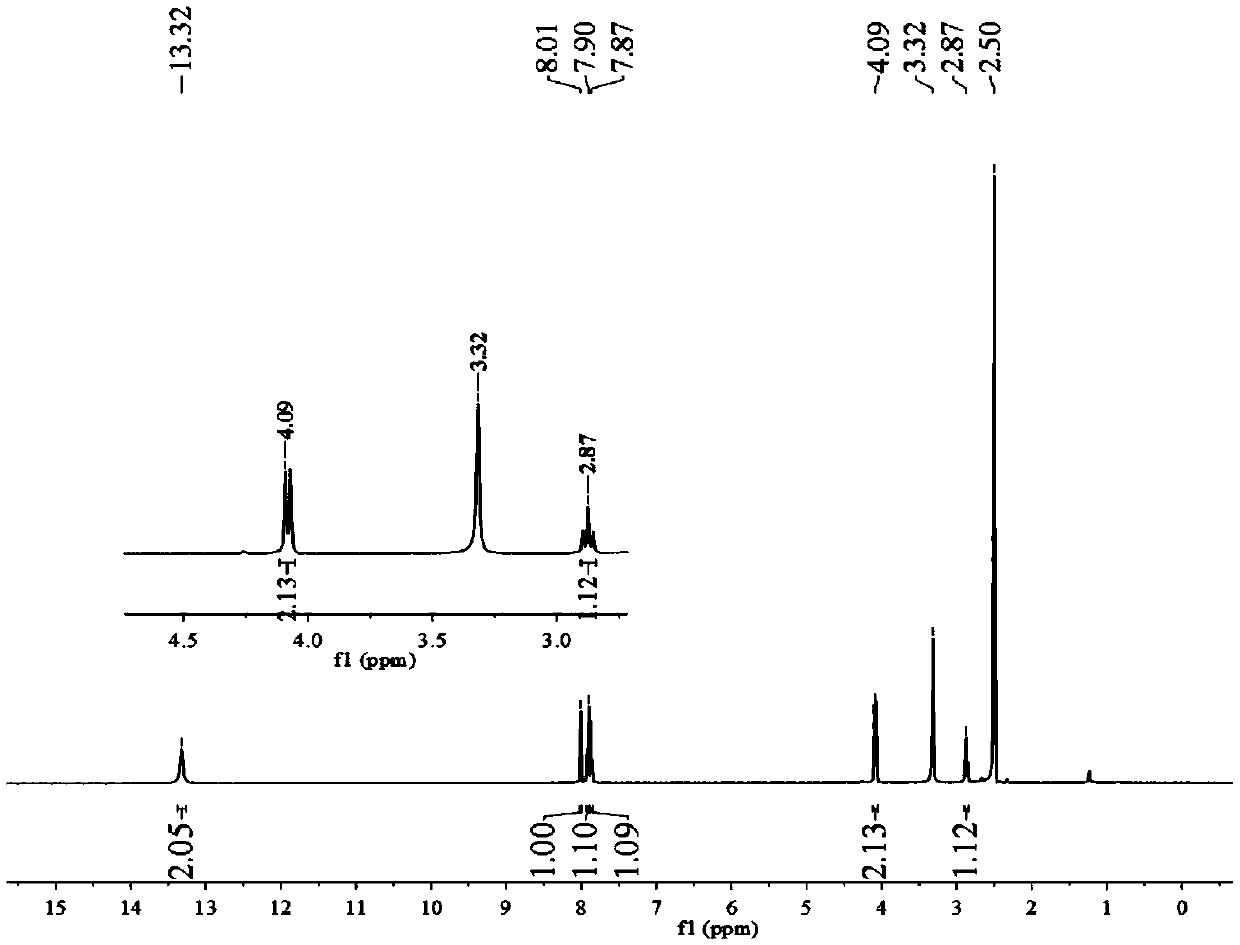
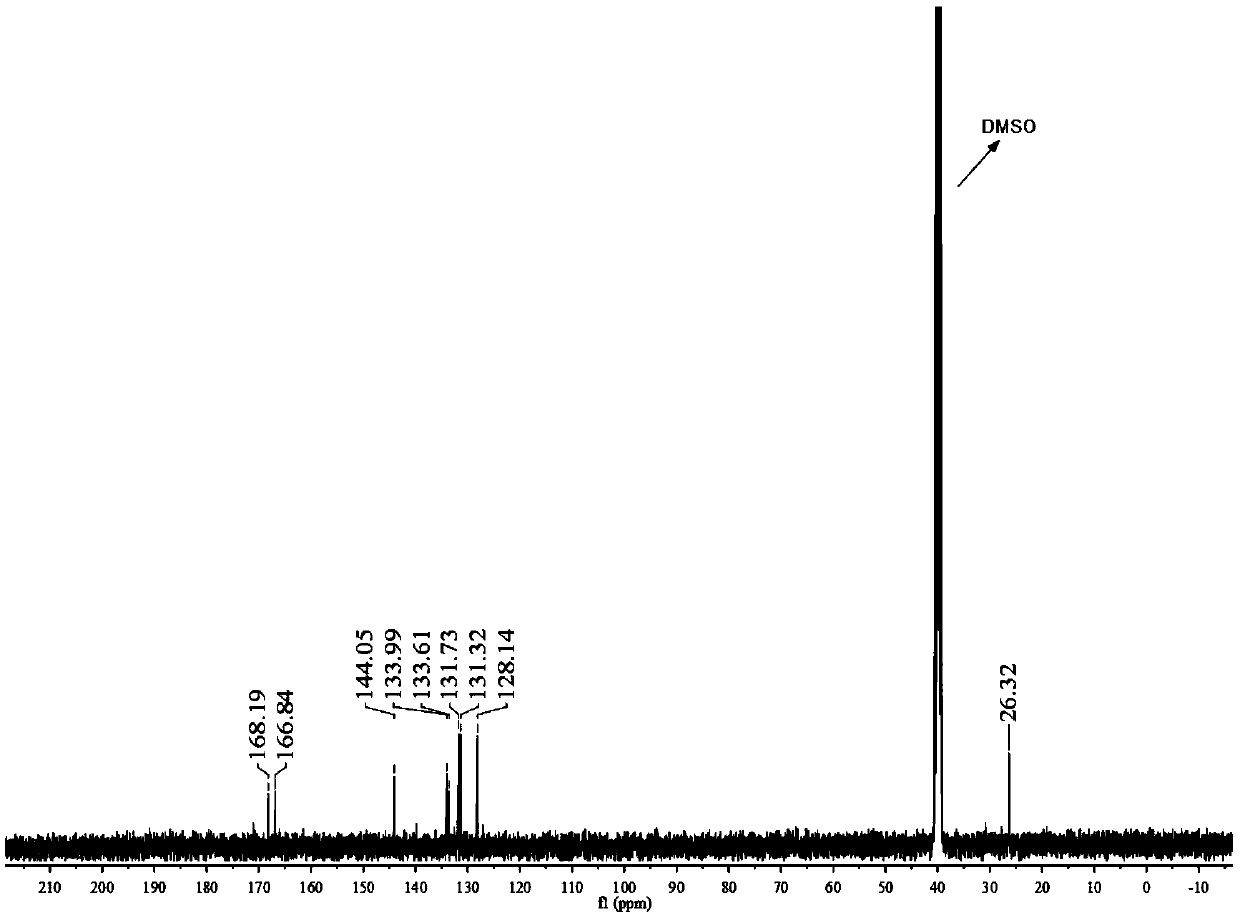
Image
Examples
preparation example Construction
[0028] The present invention provides a kind of preparation method of alkylthiol functionalized aromatic carboxylic acid compound described in the above technical scheme, comprising the following steps:
[0029] Mix 2-methylterephthalate, N-bromosuccinimide, and benzoyl peroxide under nitrogen and CCl 4 Reflux reaction under the existence of the presence of, separate reaction product, obtain 2-bromomethyl methyl terephthalate;
[0030] The 2-bromomethyl terephthalate methyl ester, sodium hydrogen sulfide and anhydrous methanol are mixed and reacted, acidified to obtain an alkyl mercaptan functionalized aromatic carboxylic acid compound.
[0031] The synthetic route of above-mentioned preparation method sees reaction scheme 1:
[0032] Reaction Scheme 1;
[0033] The present invention mixes 2-methyl terephthalate methyl ester, N-bromosuccinimide (NBS) and benzoyl peroxide (BPO), under nitrogen and CCl 4 In the presence of reflux reaction, the reaction product was separated...
Embodiment 1
[0049] One, the synthetic steps of 2-methyl terephthalate (intermediate 1):
[0050] (1) Weigh the raw material 2-methyl terephthalic acid (1801.6mg, 10mmol) into a 100mL dry single-necked round bottom flask and add a magnetic stir bar;
[0051] (2) Measure 20 mL of anhydrous methanol with a graduated cylinder and add it to a single-necked round bottom flask;
[0052] (3) Stir at room temperature and slowly add concentrated sulfuric acid (0.5mL) dropwise, then place in an oil bath to reflux for 48h. After the reaction was complete, it was cooled to room temperature, and a large amount of distilled water was added to the mixture. A large amount of solids were precipitated, and filtered under reduced pressure to obtain 1873 mg of a white solid product (i.e. Intermediate 1), with a yield of 90% and a purity of 99%.
[0053] Two, the synthetic steps of 2-bromomethyl methyl terephthalate (intermediate 2):
[0054] (1) Weigh the first intermediate (1666mg, 8mmol) prepared above, N-b...
Embodiment 2
[0073] One, the synthesis of methyl 2-methyl terephthalate
[0074] Weigh 1g of 2-methyl terephthalic acid, add 9mL of methanol, drop 40 drops of concentrated H 2 SO 4 (2mol / L) as a catalyst, reflux in an oil bath at 60-75°C for 24h. Add a large amount of deionized water to precipitate a white solid, dissolve the filtered white solid in ethyl acetate, add anhydrous MgSO 4 Water was removed, the liquid was obtained by suction filtration, and the ethyl acetate was removed in vacuo to obtain a white solid, methyl 2-methyl terephthalate.
[0075] Two, the synthesis of methyl 2-bromomethyl terephthalate
[0076] Weigh 300mg of methyl 2-methyl terephthalate (1.46mmol), add 277.5mg (1.533mmol, 1.05-1.2 times the molar amount) of NBS, 0.09-0.11 times the molar amount of BPO, 8mLCCl in anhydrous and oxygen-free operation 4 Solvent, reflux in an oil bath at 75-85°C for 6-8 hours. The insoluble solid was washed with ether and the filtrate was stripped of the solvent in vacuo to give...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com