A method for sorting and recovering heavy metals in waste nitric acid containing gold, copper and nickel
A recovery method and technology for waste nitric acid, which are applied in the improvement of process efficiency, instruments, optics, etc., to achieve the effects of high purity, good recovery rate, and easy process control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
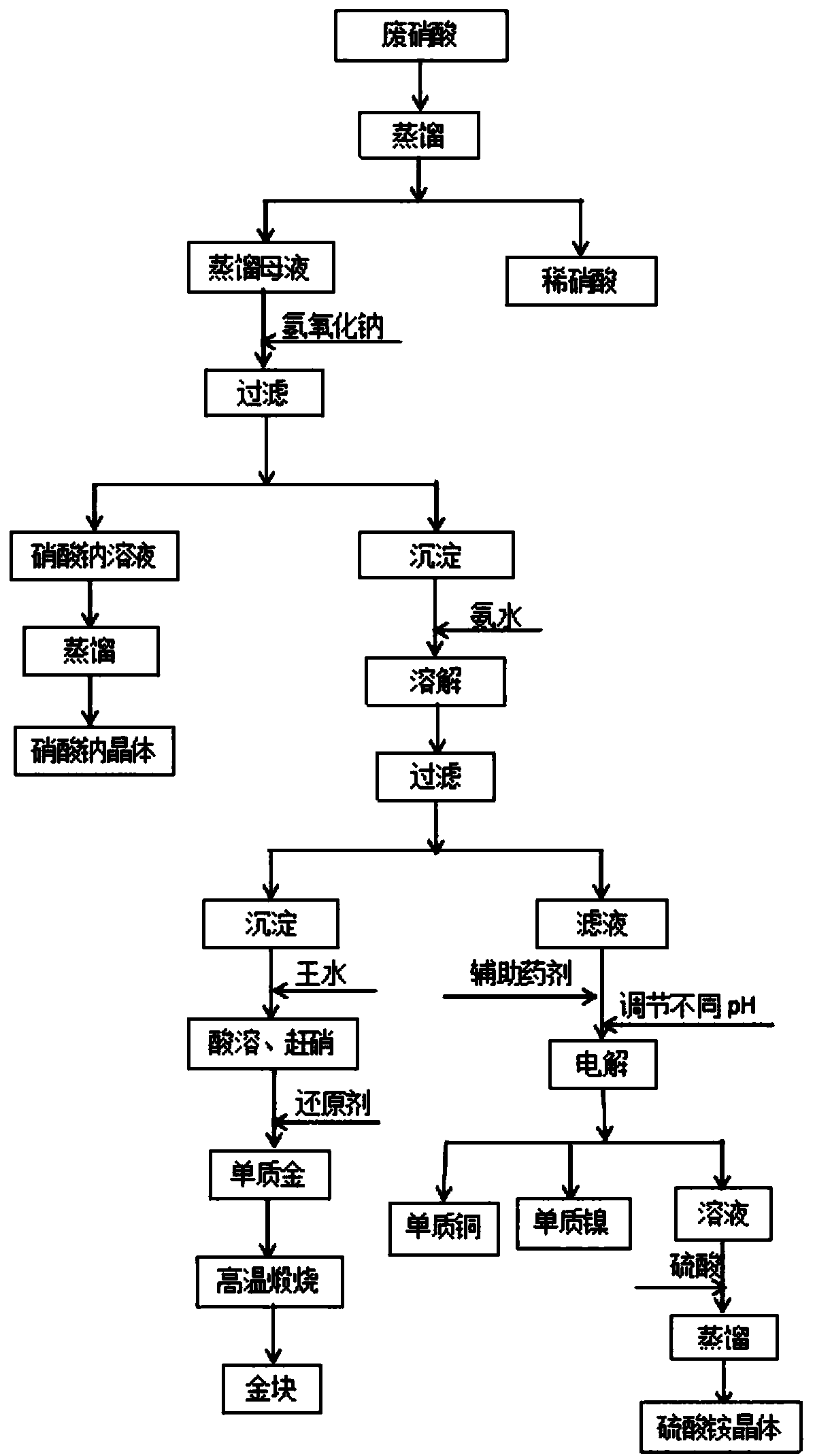
[0027] Take 1000L waste nitric acid, control the distillation pressure to be 0.04MPa, and the temperature is 100°C, carry out vacuum distillation, collect 900L dilute nitric acid, measure the acidity of dilute nitric acid to be 24.81%; add sodium hydroxide to the remaining 100L mother liquor to adjust the pH to 7.0, stir evenly, Filtrate to obtain a precipitate mainly composed of copper, nickel and gold and a filtrate mainly composed of sodium nitrate. The filtrate is controlled to distill at a pressure of 0.04MPa and a temperature of 100°C, and carry out vacuum distillation to prepare sodium nitrate crystals. The purity of the obtained sodium nitrate crystals is 99.11%;
[0028] Slowly add ammonia water to dissolve the above precipitate, then continue to add ammonia water to adjust the pH to 12, stir evenly, the total amount of ammonia water added is 10%, the total time of adding ammonia water is 2 hours, filter to obtain the filtrate mainly composed of copper and nickel and ...
Embodiment 2
[0032] Take 1500L waste nitric acid, control the distillation pressure to be 0.05MPa, and the temperature is 110°C, carry out vacuum distillation, collect 1350L dilute nitric acid, measure the acidity of dilute nitric acid to be 25.45%; add sodium hydroxide to the remaining 150L mother liquor to adjust the pH to 7.5, stir evenly, Filtrate to obtain a precipitate mainly composed of copper, nickel and gold and a filtrate mainly composed of sodium nitrate. The filtrate is controlled to distill at a pressure of 0.05MPa and a temperature of 110°C, and carry out vacuum distillation to prepare sodium nitrate crystals. The purity of the obtained sodium nitrate crystals is 99.34%;
[0033] Slowly add ammonia water to dissolve the above precipitate, then continue to add ammonia water to adjust the pH to 12.5, stir evenly, the total amount of ammonia water added is 11%, the total time of adding ammonia water is 2.5 hours, filter, and obtain filtrate mainly composed of copper and nickel an...
Embodiment 3
[0037]Take 2000L waste nitric acid, control the distillation pressure to be 0.06MPa, and the temperature is 120°C, carry out vacuum distillation, collect 1800L dilute nitric acid, measure the acidity of dilute nitric acid to be 25.22%; add sodium hydroxide to the remaining 200L mother liquor to adjust the pH to 8, stir evenly, Filtrate to obtain a precipitate mainly composed of copper, nickel, and gold and a filtrate mainly composed of sodium nitrate. The filtrate is controlled to have a distillation pressure of 0.06MPa and a temperature of 120°C, and carry out vacuum distillation to prepare sodium nitrate crystals. The purity of the obtained sodium nitrate crystals is 99.26%;
[0038] Slowly add ammonia water to dissolve the above precipitate, then continue to add ammonia water to adjust the pH to 13, stir evenly, the total amount of ammonia water added is 12%, the total time of adding ammonia water is 3 hours, filter, and obtain the filtrate mainly composed of copper and nick...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com