Periodic mesoporous organosilicon material and preparation method of polymer composite material thereof
A technology of mesoporous organosilicon and composite materials, applied in fuel cells, electrochemical generators, electrical components, etc., can solve problems such as narrow operating temperature range, and achieve the effects of low production cost, mild preparation conditions and broad application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] This embodiment relates to a method for preparing a periodic mesoporous organic silicon material, which includes the following steps:
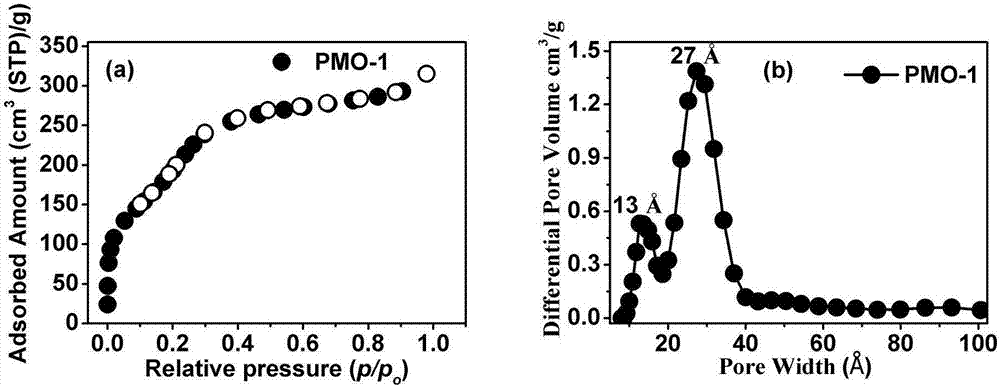
[0042]Stir octadecyltrimethylammonium chloride (3.2mmol), 6mol / L NaOH (30.4mmol), and distilled water (3.3mol) in a beaker until clear, and add 4,4'-bis(triethyl Oxysilyl)-1,1'-biphenyl (2.5mmol) was stirred at room temperature for 20h, and then the mixture was added to a 100mL polytetrafluoroethylene reactor, and the reactor was placed in a heated To 95 ℃ blast oven, react for 22h. After the reaction was over, the reactor was taken out from the drying oven and cooled to room temperature naturally. A white mixture was obtained, which was filtered with suction. The obtained filter cake is used as a solvent for Soxhlet extraction with an acidic solution of ethanol to remove the pore-forming agent in the channel. The PMO-1 material with the pore-forming agent removed was placed in a vacuum oven at 80°C for activation for 24 hours, water...
Embodiment 2
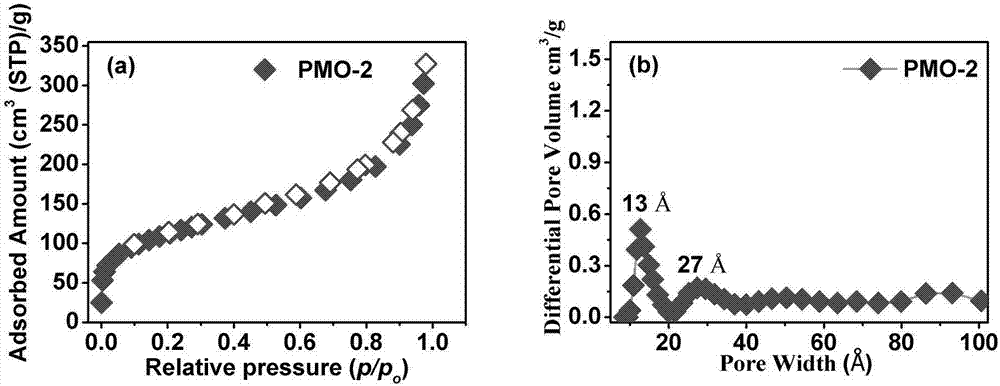
[0044] This example relates to a method for preparing a periodic mesoporous organosilicon material. The difference between this method and Example 1 is that n-octyltrimethylammonium chloride (3.2mmol) replaces octadecyltrimethyl Ammonium chloride was used as a porogen, and the resulting matrix was denoted as PMO-2. The N of the activated PMO-2 at 77K 2 The adsorption isotherm is as image 3 as shown in a. The isotherm adsorption curve of PMO-2 also shows a typical type IV curve, indicating that PMO-2 also has mesoporous characteristics and its BET specific surface area is 385m 2 / g. At the same time, nonlocal density function theory (NLDFT) also analyzes the pore size distribution of PMO-2, such as image 3 As shown in b, it shows that the pore size of PMO-2 is concentrated in and two places, but mainly in place. The activated PMO-2 was tested for proton conduction, and no semi-rings were observed in the test, indicating that the proton conductivity of the framework...
Embodiment 3
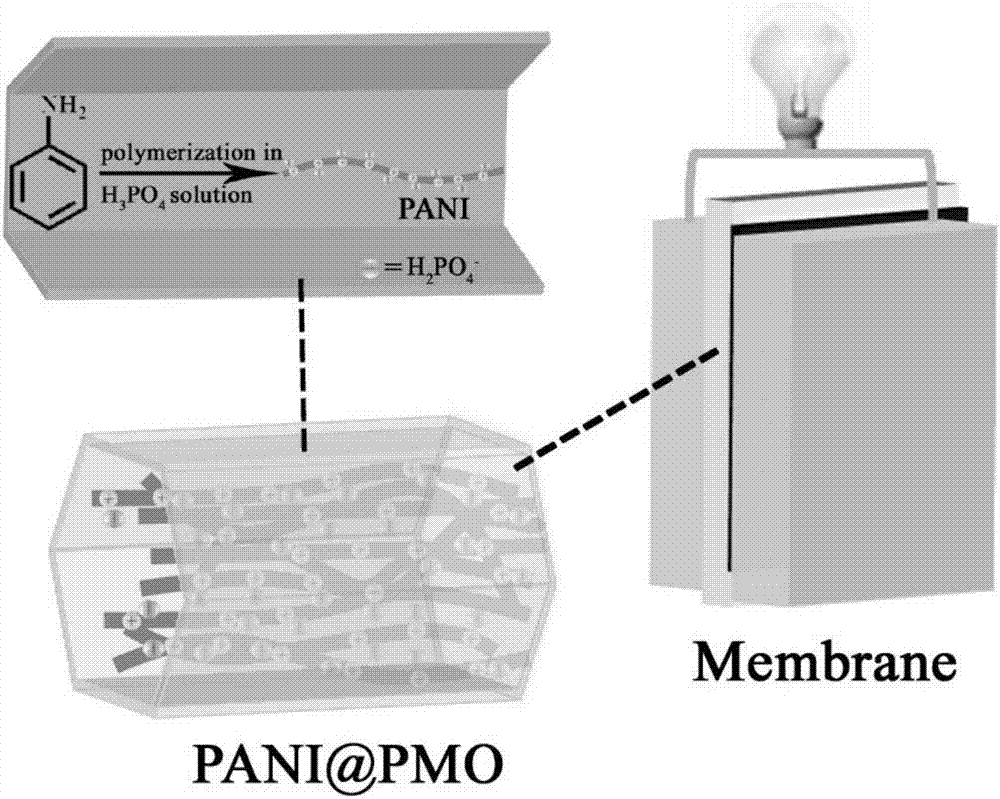
[0047] This embodiment relates to the method for preparing PANI@PMO-1 proton exchange membrane by using the PMO-1 prepared in Example 1, such as figure 1 shown, including the following steps:
[0048] Weigh 0.2g of the activated PMO-1 material, soak it in the mixed system of 8mL aniline and 10mL acetone for 24h, centrifuge, and keep the solid. Use 10 mL of phosphoric acid solution with a pH of 0.5 to transfer the above solid into a 100 mL single-necked bottle, and then place the mixed system in a water bath at -5-10°C. Weigh 0.3g of ammonium persulfate into 30mL of phosphoric acid solution with a pH of 0.5, then add this solution dropwise to the mixing system of the single-neck flask at a rate of 1mL / min, and react in a water bath at -5 to 10°C for 6h. Suction filtration, wash the filter cake with acetone until the filtrate is colorless, then place the solid in a vacuum oven at 80°C to activate for a week, remove water and solvent molecules, and obtain a low humidity dependen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com