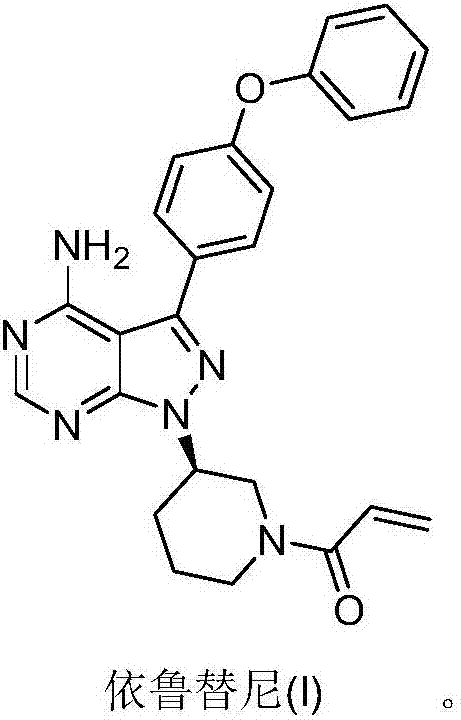
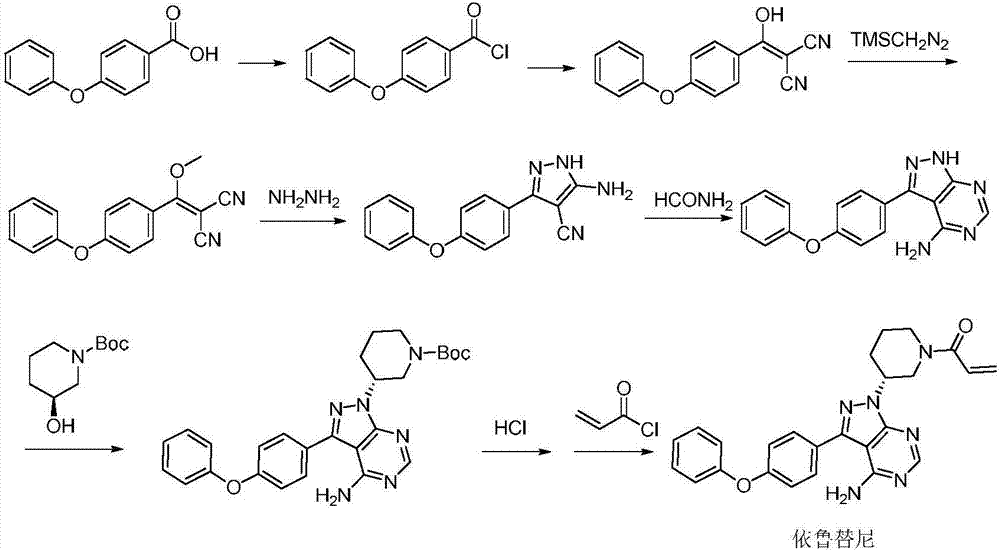
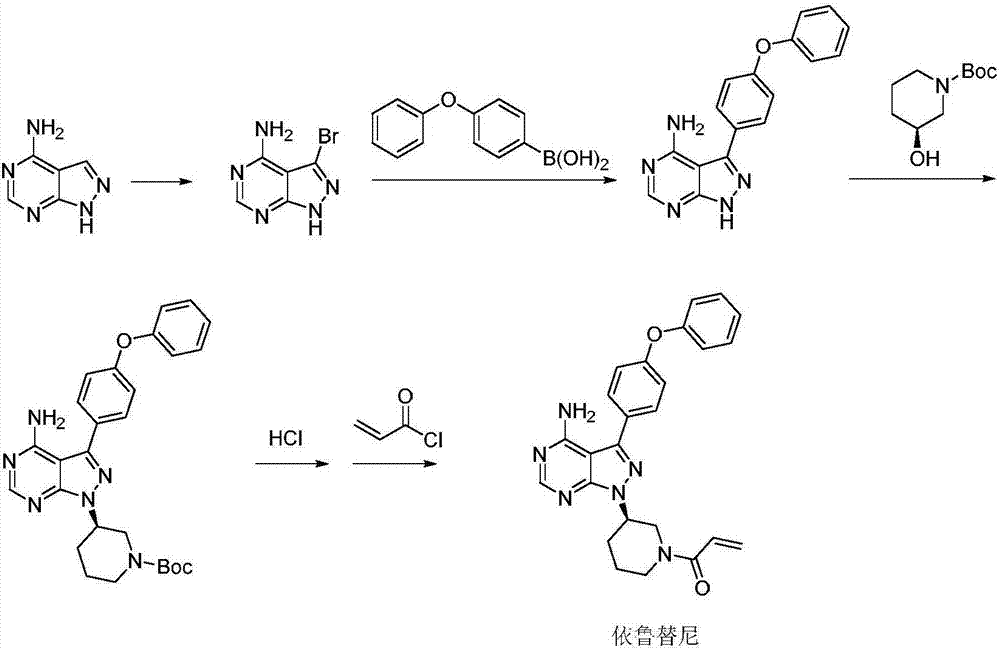
High-efficiency preparation method for ibrutinib
A high-efficiency technology for ibrutinib, applied in the field of high-efficiency preparation of ibrutinib, to achieve mild reaction conditions, simple operation, and environment-friendly effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of nitroso compound III
[0025]Mix 20.0g (200mmol) of racemic 3-aminopiperidine and 160mL of absolute ethanol in a 500mL reaction flask, add 26.0g (200mmol) of D-pyroglutamic acid, and reflux for 2 hours. At room temperature, a white solid was precipitated, filtered, and the resulting solid R-3-aminopiperidine pyroglutamate was mixed with 52.4g (240mmol) (Boc) 2 O was added to 200mL of dichloromethane, then 50.6g (500mmol) of triethylamine was added, and the reaction was stirred at room temperature for 5h. After the reaction, the solvent was concentrated, diluted with water, and then extracted with ethyl acetate. The organic phase was washed three times with a saturated NaCl solution. Concentrate under reduced pressure to obtain 29.4g product, this product, 34.2g (150mmol) periodic acid, 10g wet SiO 2 and 10.3g (150mol) of sodium nitrite were added to 200mL of dichloromethane, stirred and reacted at room temperature for 3h, after the reaction was completed...
Embodiment 2
[0027] Preparation of 3R-hydrazino-1-piperidine compound IV
[0028] Dissolve 29.6g (90mmol) of nitroso compound III in 200mL of dichloromethane, add 50mL of glacial acetic acid and 117.0g of zinc powder, and react at room temperature for 15min. After the reaction, add saturated NaCO 3 The solution adjusts the pH of the reaction system to be alkaline, adding ethyl acetate for extraction, standing still, layering, pouring out the lower aqueous phase, and then refilling the organic phase with saturated NaCO 3 The solution was washed once, and finally washed three times with a saturated NaCl solution, the organic phase was concentrated, and 23.6 g of a hydrazine-based intermediate was obtained through chromatography column purification, and then the hydrazine-based intermediate was added to 90 mL of 4M hydrogen chloride in methanol solution, and the reaction was stirred at room temperature for 0.5 h. The solvent was evaporated to dryness to obtain 13.5 g of 3R-hydrazino-1-piperid...
Embodiment 3
[0030] Preparation of 3R-hydrazino-1-piperidine compound IV
[0031] Dissolve 29.6g (90mmol) of nitroso compound III in 200mL of dichloromethane, add 50mL of glacial acetic acid and 117.0g of zinc powder, and react at room temperature for 10min. After the reaction, add saturated NaCO 3 The solution adjusts the pH of the reaction system to be alkaline, adding ethyl acetate for extraction, standing still, layering, pouring out the lower aqueous phase, and then refilling the organic phase with saturated NaCO 3 The solution was washed once, and finally washed three times with saturated NaCl solution, the organic phase was concentrated, and 23.6 g of hydrazine-based intermediates were obtained through chromatography column purification, and then the hydrazine-based intermediates were added to 180 mL of 4M hydrogen chloride in methanol solution, and the reaction was stirred at 10 ° C After 2 hours, the solvent was evaporated to dryness to obtain 10.2 g of 3R-hydrazino-1-piperidine c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com