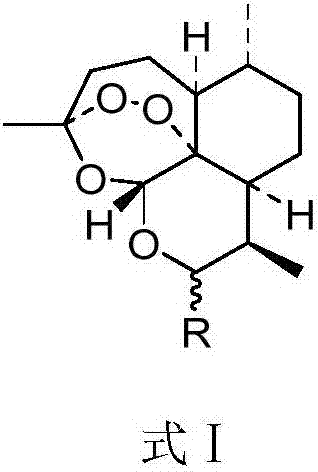
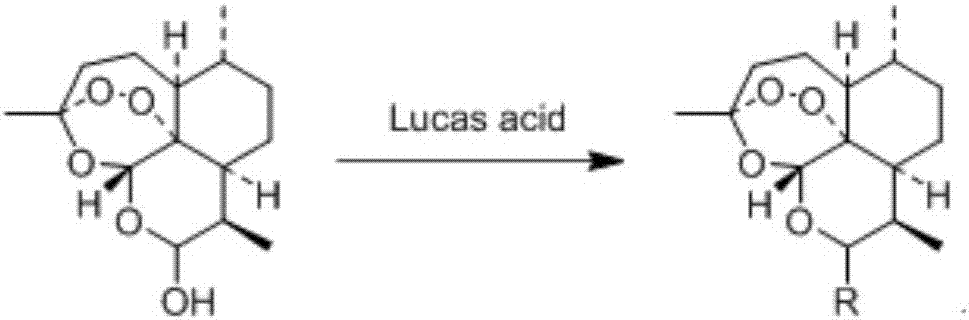
Artemisinin derivative as well as synthesis and application thereof
A technology for artemisinin and derivatives, which is applied in the field of drug synthesis, can solve the problems of difficulty in producing specific vaccines, threats to human health, and no effective treatment methods, and achieves simple and easy preparation methods, low environmental pollution, and reproducibility. Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
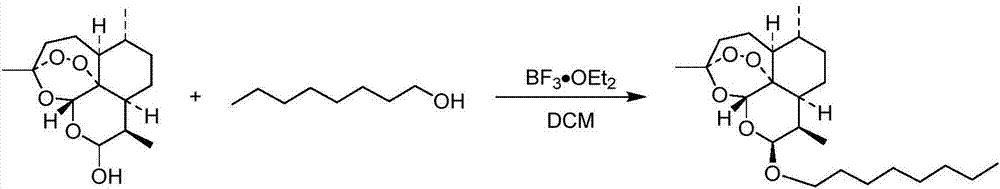
[0044] Embodiment 1: Preparation of KPC-4000055
[0045]
[0046] Dissolve dihydroartemisinin (300mg, 1.06mmol, 1.0eq) and n-octanol (331mmL, 2.11mmol, 2.0eq) in a 10mL three-necked flask filled with 5mL of dichloromethane, replace nitrogen, and cool to -10 Add boron trifluoride diethyl ether (53mmL, 422μmol, 0.4eq) dropwise at °C and stir at this temperature for 2h. TLC (ethyl acetate:petroleum ether=2:8) showed that the reaction was complete. Slowly pour the reaction solution into water to quench, extract 3 times with ethyl acetate, combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, filter, remove the solvent on the rotary evaporation to obtain the crude product, pass through the column layer Purification by analysis (ethyl acetate:petroleum ether=2:8) gave colorless oily liquid KPC-4000055 (320 mg, yield 76%).
[0047] KPC-4000055: 1 H NMR (800MHz, CHLOROFORM-d) δ: 5.39(s, 1H), 4.78(d, J=3.3Hz, 1H), 3.83(td, J=6.6, 9.6Hz, 1H), 3....
Embodiment 2
[0050] Embodiment 2: Preparation of KPC-4000056
[0051]
[0052] Dihydroartemisinin (1.0g, 3.52mmol, 1.0eq) and 1,4-butanediol (3.11mL, 35.17mmol, 10.0eq) were dissolved in a 25mL three-necked flask containing 10mL of dichloromethane, Nitrogen was replaced, the temperature was lowered to 5°C, and boron trifluoride diethyl ether (442mmL, 3.52mmol, 1.0eq) was added dropwise, and stirred at this temperature for 2h. TLC (ethyl acetate:petroleum ether=4:6) showed that the reaction was complete. Slowly pour the reaction solution into water to quench, extract 3 times with ethyl acetate, combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, filter, remove the solvent on the rotary evaporation to obtain the crude product, pass through the column layer Purification by analysis (ethyl acetate:petroleum ether=4:6) gave white solid KPC-4000056 (1.1 g, yield 88%).
[0053] KPC-4000056: 1 H NMR (800MHz, CHLOROFORM-d) δ: 5.39 (d, J=2.2Hz, 1H), 4.79 (...
Embodiment 3
[0056] Embodiment 3: Preparation of KPC-4000057
[0057]
[0058] Dissolve dihydroartemisinin (399mg, 1.40mmol, 1.0eq) and KPC-4000056 (500mg, 1.40mmol, 1.0eq) in a 25mL three-necked flask filled with 10mL of dichloromethane, replace nitrogen, and cool down to - Boron trifluoride diethyl ether (178mmL, 1.40mmol, 1.0eq) was added dropwise at 20°C and stirred at this temperature for 2h. TLC (ethyl acetate:petroleum ether=4:6) showed that the reaction was complete. Slowly pour the reaction solution into water to quench, extract 3 times with ethyl acetate, combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, filter, remove the solvent on the rotary evaporation to obtain the crude product, pass through the column layer Purification by analysis (ethyl acetate:petroleum ether=4:6) gave white solid KPC-4000057 (650 mg, yield 76%).
[0059] KPC-4000057: 1H NMR (800MHz, CHLOROFORM-d) δ: 5.38(s, 1H), 5.33(s, 1H), 4.77(d, J=3.4Hz, 1H), 4.41(d, J=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com