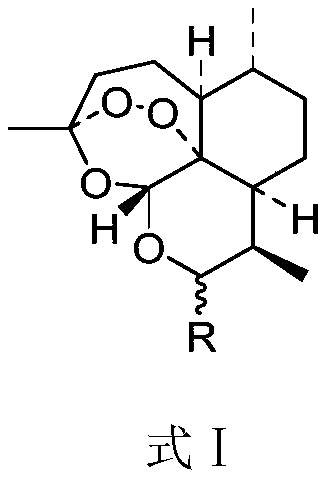
A kind of artemisinin derivative, its synthesis and application
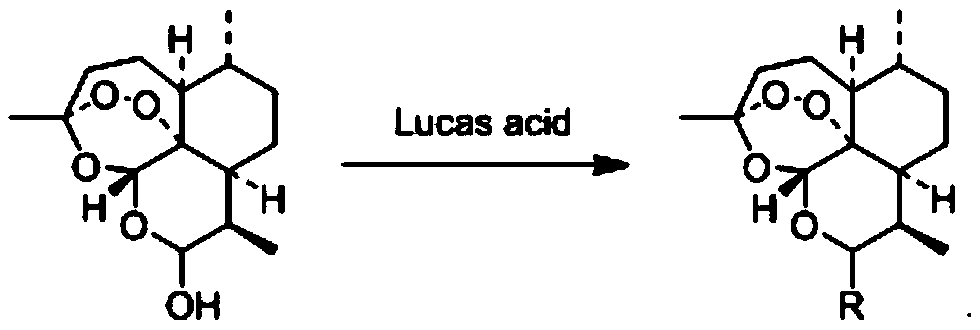
A derivative, artemisinin technology, applied in the field of drug synthesis, can solve the problems of no effective treatment method, difficulty in producing specific vaccines, human health threats, etc., and achieves simple and easy preparation method, good reproducibility, and environmental pollution. small effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
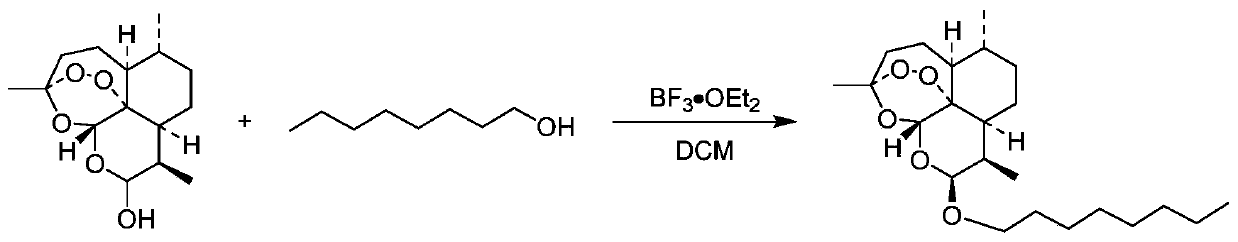
[0044] Embodiment 1: Preparation of KPC-4000055
[0045]
[0046] Dissolve dihydroartemisinin (300mg, 1.06mmol, 1.0eq) and n-octanol (331mmL, 2.11mmol, 2.0eq) in a 10mL three-necked flask filled with 5mL of dichloromethane, replace nitrogen, and cool to -10 Add boron trifluoride diethyl ether (53mmL, 422μmol, 0.4eq) dropwise at °C and stir at this temperature for 2h. TLC (ethyl acetate:petroleum ether=2:8) showed that the reaction was complete. The reaction solution was slowly poured into water to quench, extracted 3 times with ethyl acetate, the organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and the solvent was removed on a rotary evaporator to obtain a crude product, which was passed through the column layer Purification by analysis (ethyl acetate:petroleum ether=2:8) gave colorless oily liquid KPC-4000055 (320 mg, yield 76%).
[0047] KPC-4000055: 1 H NMR (800MHz, CHLOROFORM-d) δ: 5.39(s, 1H), 4.78(d, J=3.3Hz,...
Embodiment 2
[0050] Embodiment 2: Preparation of KPC-4000056
[0051]
[0052] Dihydroartemisinin (1.0g, 3.52mmol, 1.0eq) and 1,4-butanediol (3.11mL, 35.17mmol, 10.0eq) were dissolved in a 25mL three-necked flask containing 10mL of dichloromethane, Nitrogen was replaced, the temperature was lowered to 5°C, and boron trifluoride diethyl ether (442mmL, 3.52mmol, 1.0eq) was added dropwise, and stirred at this temperature for 2h. TLC (ethyl acetate:petroleum ether=4:6) showed that the reaction was complete. The reaction solution was slowly poured into water to quench, extracted 3 times with ethyl acetate, the organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and the solvent was removed on a rotary evaporator to obtain a crude product, which was passed through the column layer Purification by analysis (ethyl acetate:petroleum ether=4:6) gave white solid KPC-4000056 (1.1 g, yield 88%).
[0053] KPC-4000056: 1 H NMR (800MHz, CHLOROFORM...
Embodiment 3
[0056] Embodiment 3: Preparation of KPC-4000057
[0057]
[0058] Dissolve dihydroartemisinin (399mg, 1.40mmol, 1.0eq) and KPC-4000056 (500mg, 1.40mmol, 1.0eq) in a 25mL three-neck flask filled with 10mL of dichloromethane, replace nitrogen, and cool down to - Boron trifluoride diethyl ether (178mmL, 1.40mmol, 1.0eq) was added dropwise at 20°C and stirred at this temperature for 2h. TLC (ethyl acetate:petroleum ether=4:6) showed that the reaction was complete. The reaction solution was slowly poured into water to quench, extracted 3 times with ethyl acetate, the organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and the solvent was removed on a rotary evaporator to obtain a crude product, which was passed through the column layer Purification by analysis (ethyl acetate:petroleum ether=4:6) gave white solid KPC-4000057 (650 mg, yield 76%).
[0059] KPC-4000057: 1H NMR (800MHz, CHLOROFORM-d) δ: 5.38(s, 1H), 5.33(s, 1H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com