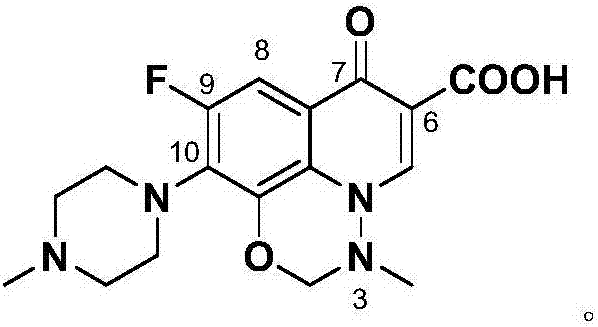
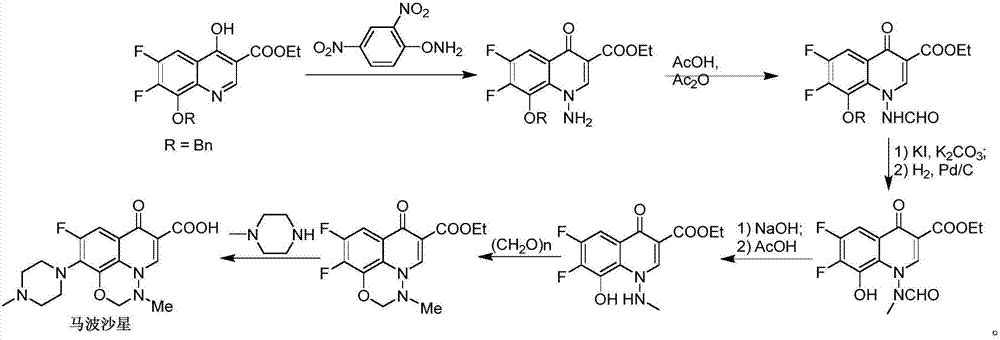
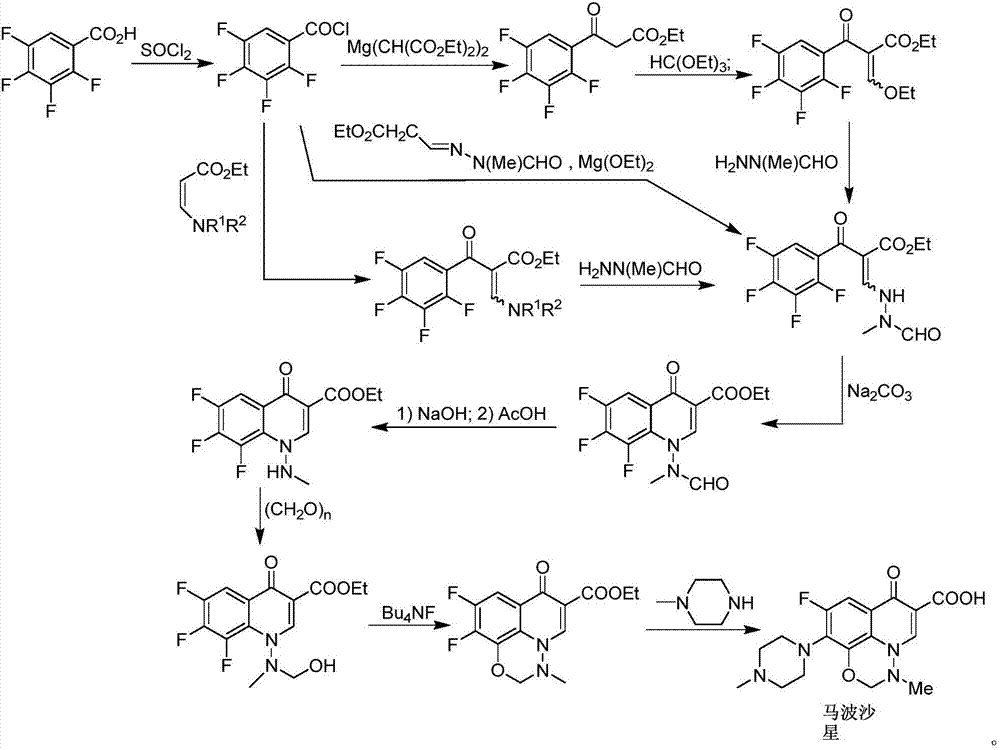
Marbofloxacin preparation method
A technology for marbofloxacin and compound, which is applied in the field of medicinal chemistry, can solve the problems of unfavorable chemical reagents, excessively long reaction route, expensive chemical reagents and the like, and achieves the effects of novel synthesis method, high efficiency and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Preparation of 1,1,1-trichloro-4-(4-methylpiperazin-1-yl)but-3-en-2-one
[0027] (E)-1,1,1-trichloro-4-methoxybut-3-en-2-one (formula I, R=Me) (10.18g, 50mmol), 1-methylpiperazine (6.0 g, 60mmol) and mesitylene (50mL) was heated to reflux temperature and stirred for 6 hours, the system was naturally cooled to room temperature, the organic solvent was removed under high vacuum and reduced pressure, and the residue (14.2g, crude product, unpurified) was untreated purified and used directly in the next reaction.
Embodiment 2
[0028] Example 2: (6,8-difluoro-7-(4-methylpiperazin-1-yl)-4-oxo-3-(2,2,2-trichloroacetyl)quinoline-1 Preparation of (4H)-yl) ethyl carbamate (formula VII)
[0029] Under nitrogen protection, the product of Example 1 (14.2 g, not purified, directly used) was dissolved in toluene (120 mL), then triethylamine (72 mL, 514 mmol) was added to the system, and the system was heated to reflux temperature. At reflux temperature, a solution of 2,3,4,5-tetrafluorobenzoyl chloride (16 g, 75.3 mmol) in toluene (60 mL) was slowly added dropwise to the reaction system. After the dropwise addition, the system was refluxed for 30 minutes, and then the system was slowly cooled to 60°C, and filtered hot. Filtrate is transferred in the 500ml reaction bottle, then adds ethyl carbazate (formula V, R 2 = Et) (6.25 g, 60 mmol). After the addition, the system was reacted at a temperature of 60-65° C. for 12 hours. Slowly add H to the reaction system 2 O (150 mL) was used to quench the reaction, a...
Embodiment 3
[0030] Example 3: 1-amino-6-fluoro-8-hydroxyl-7-(4-methylpiperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid ( Preparation of formula VIII)
[0031] The solid obtained in Example 2 (21.2g) was placed in a 200ml reaction flask, ethanol (50mL) and water (50mL) were added to the reaction system, and the system was heated to reflux. Under the condition of reflux, an aqueous solution (30 mL) of KOH (7.0 g) was slowly added to the system, and after the dropwise addition, the system was kept under reflux for 96 hours. The system was naturally cooled to room temperature, and the system was added with H 2 O (100mL) and CH 2 Cl 2 (50ml), after stirring, let it stand to separate the organic phase, and then use CH 2 Cl 2 Extract twice (2 x 50 mL). Use hydrochloric acid to adjust the system to acidity (pH=3-4) in the water phase, and then use CH 2 Cl 2 Extracted twice (2×100 mL), combined the organic phases, and removed the organic solvent under reduced pressure to obtain ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com