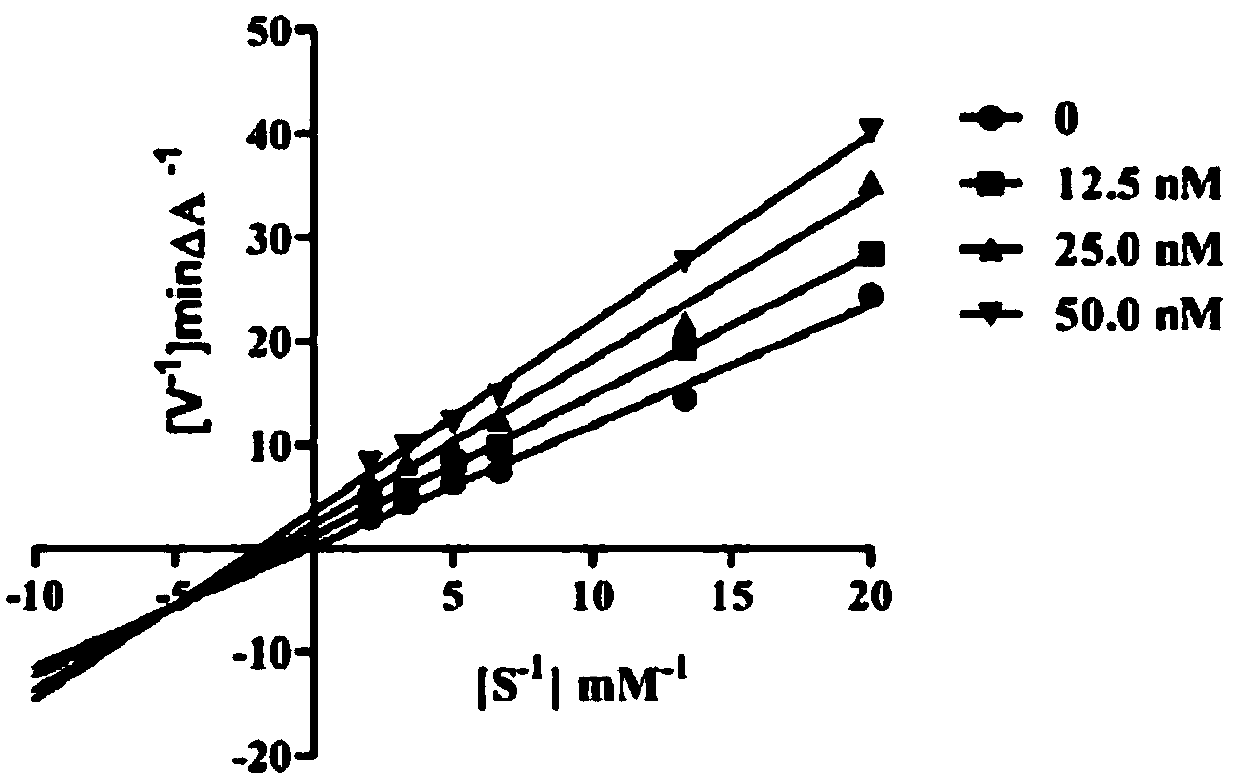
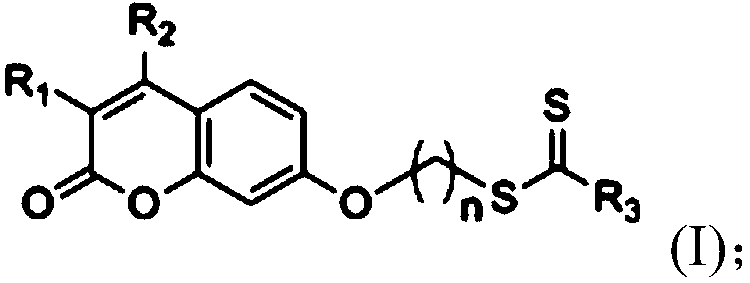
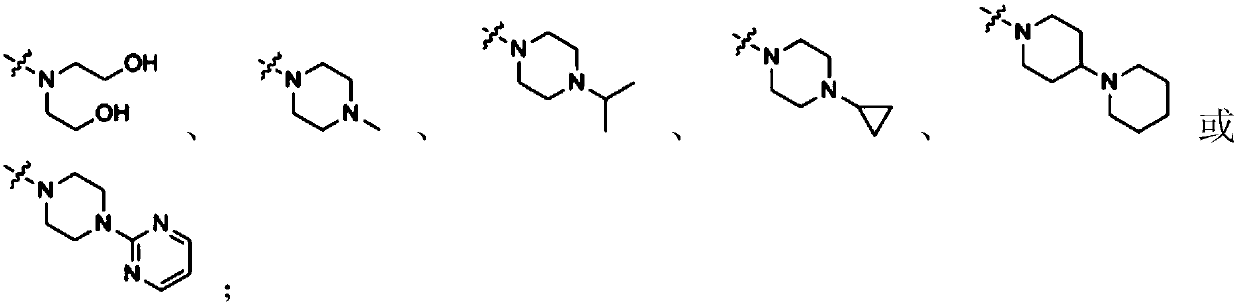
Coumarin-dithiocarbamate derivative and synthesizing method thereof
A synthetic method and dimethylformamide technology, applied in the field of coumarin-dithiocarbamate derivatives and their synthesis, can solve the problem of undiscovered coumarin-dithiocarbamate derivatives The synthetic method cannot fundamentally improve the disease state, terminate the disease and other problems, and achieve the effect of easy operation, high yield and stable quality of the synthetic method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1: the preparation of intermediate 2 (7-hydroxyl-4-methylcoumarin)
[0051] Measure 15ml of dioxane (which can also be methanol or ethanol), add resorcinol (10mmol, 1.1g) to it, and add 4ml of concentrated sulfuric acid dropwise under ice bath conditions. Add ethyl acetoacetate (10mmol, 1.3g) into the solution, stir and heat to 60°C, and react for 4h. Recrystallized from methanol to obtain white needle-like solid crystals with a yield of 91%. 1 H NMR (600MHz, acetone-d 6 )δ7.61 (1H, d, J = 9.0Hz), 6.85 (1H, dd, J = 9.0, 2.5Hz), 6.75 (1H, d, J = 2.5Hz), 6.09 (1H, s), 2.40 ( 3H,s).ESI-MS m / z:175.0[M-H] - .
Embodiment 2
[0052] Example 2: General procedure for the preparation of intermediates 3a-f
[0053] Add 5.0mmol of intermediate 2 and 50mmol of dibromoalkane while stirring in acetone (also can be DMF, acetonitrile or tetrahydrofuran), and then add anhydrous K 2 CO 3 (1.4g, 10mmol) to adjust the pH of the system to 9-11, react at room temperature for 4 hours, then filter with suction after cooling, collect the filter residue, and purify the filter residue through a silica gel column (petroleum ether:ethyl acetate=15:1, volume ratio) , finally affording 3a-f as white solids.
Embodiment 3
[0054] Embodiment 3: The yield of intermediate 7-(8-bromoethoxy)-4-methylcoumarin (3a), NMR data and mass spectrometry data
[0055] Yield 86%; 1 H NMR (600MHz, CDCl 3 )δ7.53(d, J=8.8Hz, 1H), 6.91(dd, J=8.8, 2.5Hz, 1H), 6.83(d, J=2.5Hz, 1H), 6.17(s, 1H), 4.37( t,J=6.1Hz,2H),3.70(t,J=6.1Hz,2H),2.40(s,3H); ESI-MS m / z:283.1[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com