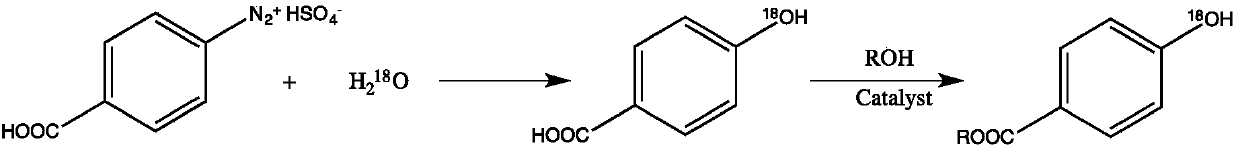
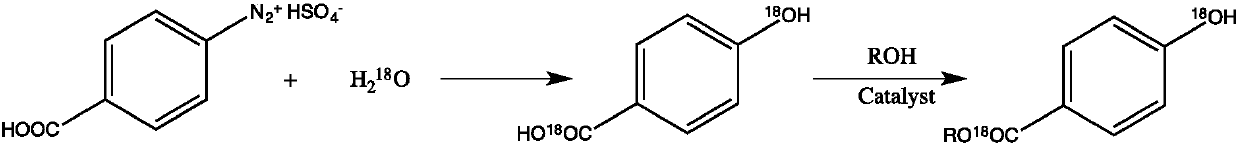
Synthesis method of stable isotope 18O labeled nipagin ester
A paraben ester and synthesis method technology, applied in the direction of isotope introduction of organic compounds, acyclic/carbocyclic compound isotope introduction, organic chemical methods, etc., can solve neonatal genital deformities, paraben residues, increase female uterus Cancer risk and other issues, to achieve the effect of small dilution, high utilization rate, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] a stable isotope 18 The synthetic method of the methylparaben of O mark adopts the following steps:
[0024] (1) In a 100ml glass three-neck flask, add 18.45g (0.075mol) of benzoic acid-4-diazosulfate and 50mL of anhydrous tetrahydrofuran, control the reaction temperature at 0°C, and add slowly under stirring 18 O labeled water 0.9g (0.05mol), reacted at low temperature for 4h, then heated the oil bath to 60°C for 2h, then stirred at room temperature for 4h, directly evaporated the solvent under reduced pressure, and separated by silica gel column chromatography to obtain 6.3g of intermediate product nipol Auric acid- 18 O.
[0025] (2) In a 100ml glass three-necked bottle, add the above-mentioned Nipaginic acid- 18 O and 25mL of methanol, in the process of stirring, slowly add 4.4g of concentrated sulfuric acid as a catalyst, then the oil bath is heated to reflux, after 8 hours of reaction, it is lowered to room temperature, the solvent is evaporated under reduced p...
Embodiment 2
[0027] a stable isotope 18 The synthetic method of the propylparaben of O mark adopts the following steps:
[0028] (1) In a 100ml glass three-necked flask, add 24.6g (0.1mol) of benzoic acid-4-diazosulfate and 50mL of anhydrous 2-methyltetrahydrofuran, control the reaction temperature at 5°C, and add slowly under stirring 18 O marked water 0.9g (0.05mol), reacted at low temperature for 6h, then heated the oil bath to 80°C for 4h, then stirred at room temperature for 8h, directly evaporated the solvent under reduced pressure, and then separated by silica gel column chromatography to obtain 6.1g of intermediate product Ni Parkinic acid- 18 O.
[0029] (2) In a 100ml glass three-necked bottle, add the above-mentioned Nipaginic acid- 18 O and 25mL propanol, in the process of stirring, slowly add 7.5g p-toluenesulfonic acid as a catalyst, then the oil bath is heated to reflux, after 6 hours of reaction, it is lowered to room temperature, the solvent is evaporated to dryness und...
Embodiment 3
[0031] a stable isotope 18 The synthetic method of the O-marked octylparaben adopts the following steps:
[0032] (1) In a 100ml glass three-necked flask, add 36.9g (0.15mol) of benzoic acid-4-diazosulfate and 50mL of anhydrous 1,4-dioxane, control the reaction temperature at 5°C, and add dropwise under stirring slow 18 O labeled water 0.9g (0.05mol), reacted at low temperature for 8h, then heated the oil bath to 80°C for 6h, then stirred at room temperature for 6h, directly evaporated the solvent under reduced pressure, and then separated by silica gel column chromatography to obtain 6.0g intermediate product Ni Parkinic acid- 18 O.
[0033] (2) In a 100ml glass three-necked bottle, add the above-mentioned Nipaginic acid- 18 O, add octanol 24.7g (0.21mol) and 25mL toluene again, in stirring process, slowly add the concentrated sulfuric acid of 4.1g as catalyst, then oil bath is warmed up to reflux, and utilizes water divider to divide water, after reaction 12h, drop to A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com