Water-soluble IDO (dioxygenase) inhibitor cisplatin and preparation method thereof
An inhibitor, water-soluble technology, applied in chemical instruments and methods, drug combinations, pharmaceutical formulations, etc., can solve problems such as poor water-soluble immunosuppression, and achieve the effect of maintaining the killing effect, increasing the effect of immunotherapy, and improving the environment of immunosuppression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Weigh 200mg (0.53mmol) of Pt(1R,2R-DACH)Cl 2 , 88.47mg (0.52mmol) of silver nitrate and 151.53mg (0.54mmol) of NLG919 were dissolved in 20mL of anhydrous DMF, protected by argon, protected from light, and reacted at 55°C for 24h. After the reaction, the reaction solution was lowered to room temperature, centrifuged at room temperature 3000rpm for 5min, the supernatant was taken, concentrated to about 1mL by rotary evaporation, and column chromatography was carried out with chloroform:methanol:acetone:glacial acetic acid=10:0.5:4:0.1 , take compounds with Rf=0.2-0.3, combine and concentrate to obtain white or light yellow solid, dry in vacuum at 40°C, and weigh to obtain 145.55 mg of the product (yield 44.11%). And the product is soluble in water, the solubility can reach 2.85mg / mL.
[0023] The chemical structure of the compound synthesized in this example is shown in Formula 1.
[0024]
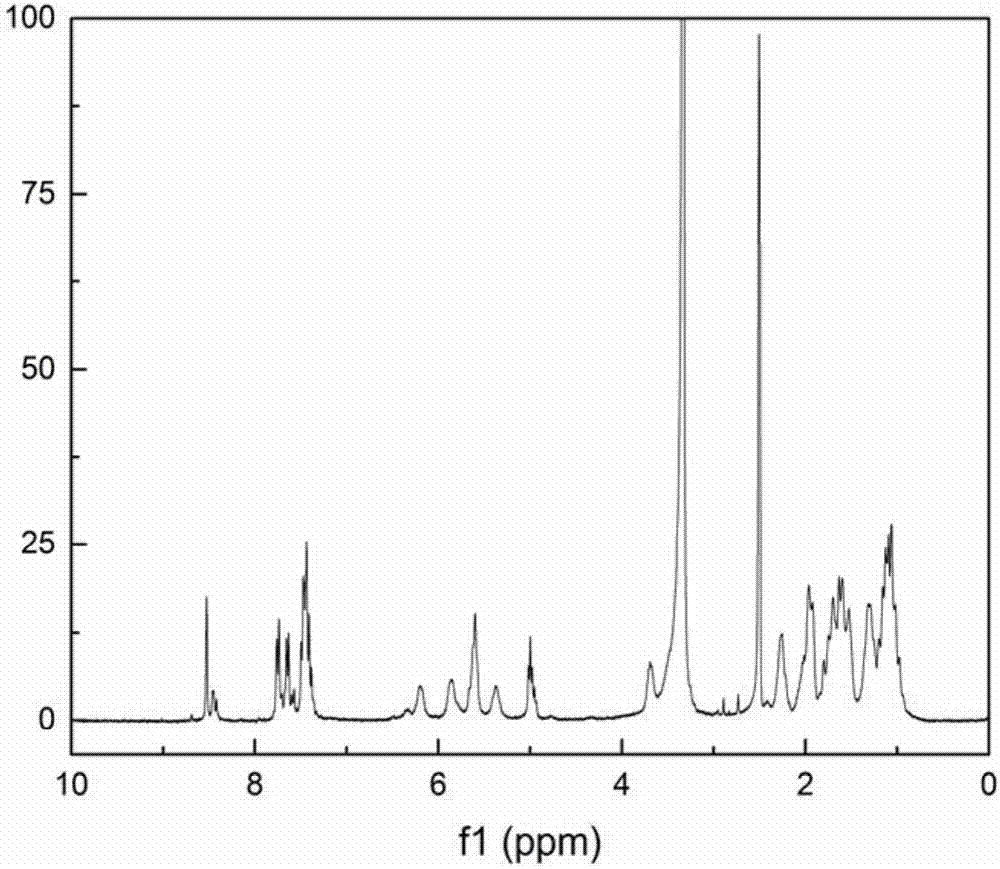
[0025] of the conjugates prepared in this example 1 H NMR spectrum as figu...
Embodiment 2
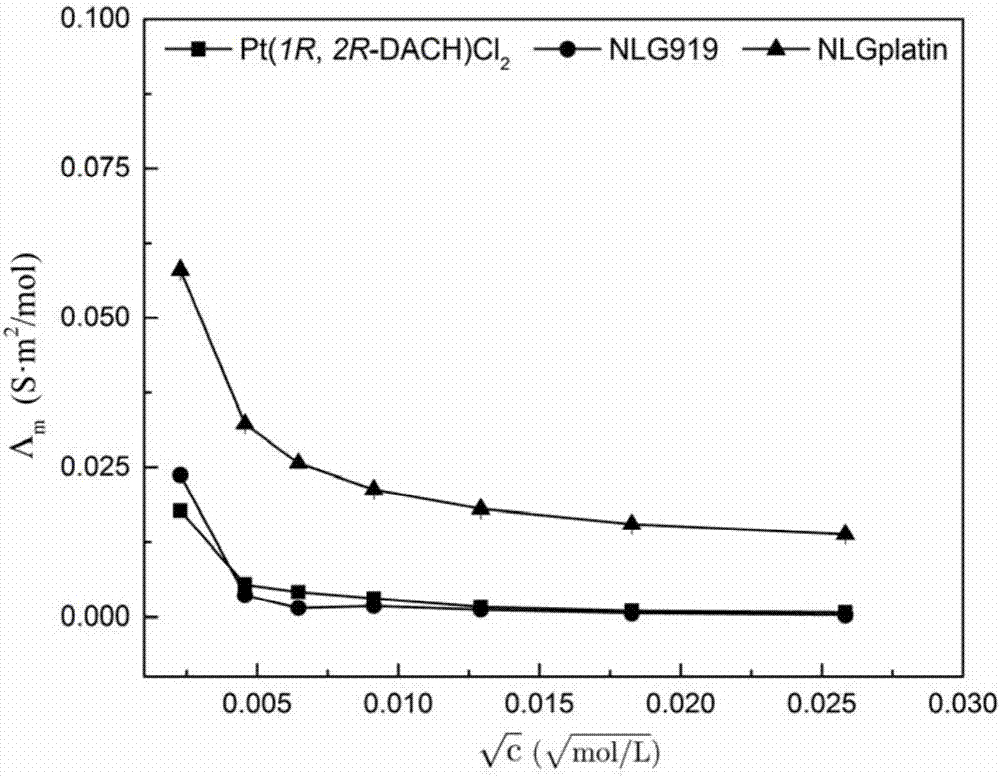
[0028] In the state of conductivity measurement, insert the clean and rinsed electrode and temperature sensor into 0.0100mol / L KCl solution (about 20mL), calculate according to the value, get the actual electrode constant value, and input the actual electrode constant value into the instrument middle. Take 20mL of distilled water in a 50mL beaker and measure its conductivity. Determination of Pt(1R,2R-DACH)Cl with different concentration gradients 2 , NLG919, [Pt(1R,2R-DACH)C 18 h 22 N 2 O]Cl NO 3 Conductivity, the concentration gradient is 5.22, 20.86, 74.72, 83.44, 166.88, 333.76, 667.52μM.
[0029] The molar conductivity-concentration data that the present embodiment measures is as image 3 shown.
Embodiment 3
[0031] Determination of [Pt(1R,2R-DACH)C by MTT method 18 h 22 N 2 O]Cl NO 3 For the inhibitory effect on tumor cell viability, the in vitro cell viability inhibition experiment was carried out in 4T1 cells and HeLa cells. The specific operation steps are as follows: first, 4T1 and HeLa cells in the logarithmic growth phase were added to 96-well plates, and the plating density per well was is 1×10 4 / 200μL, cultivated in the cell culture incubator for 24h. Then the oxaliplatin group, NLG919 group, [Pt(1R,2R-DACH)C 18 h 22 N 2 O]Cl NO 3group, oxaliplatin and NLG919 physical mixing group were diluted into a series of concentration gradients, and the cells that were not given drugs were used as the control group, and the cells that were only added with medium in the blank wells were used as the blank group. The drug concentrations administered to 4T1 and HeLa cells were 1, 1.6, 2.8, 4.7, 7.8, 13.0, 21.6, 36.0, 60.0, and 100.0 μM, respectively, based on oxaliplatin. Aspir...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com