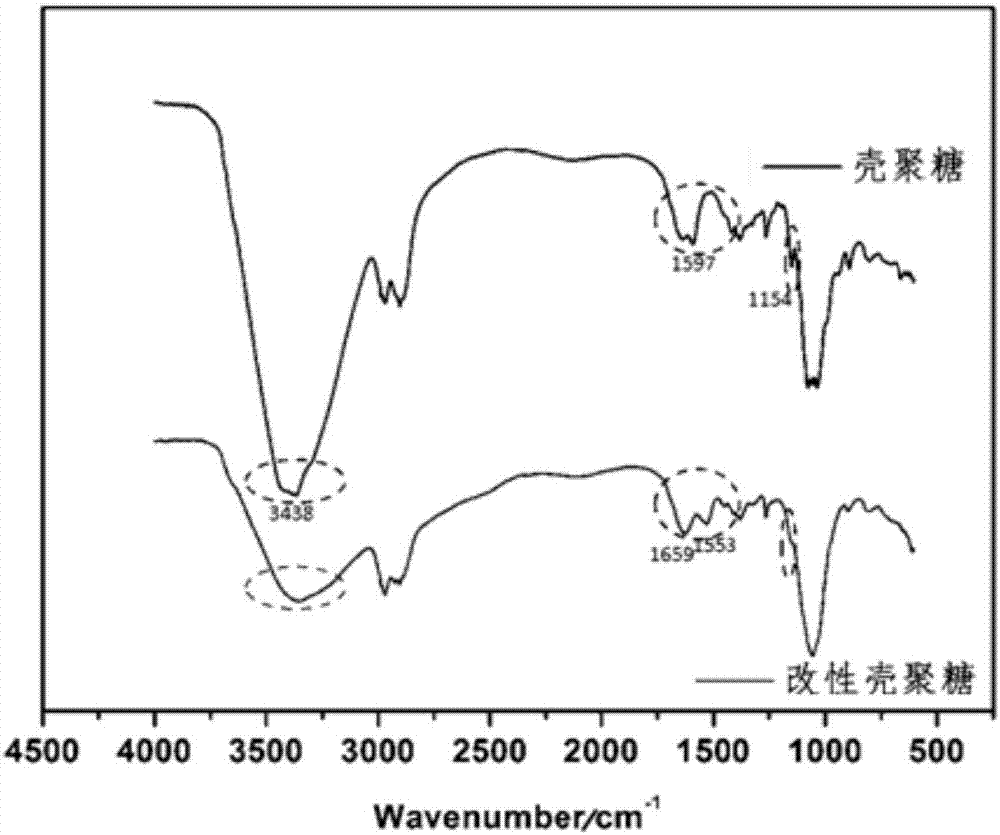
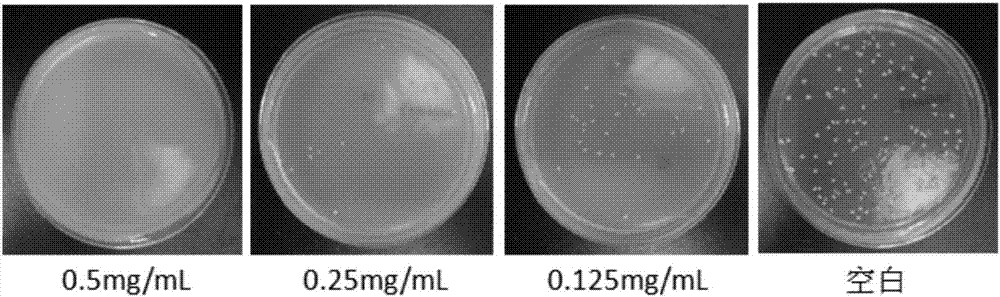
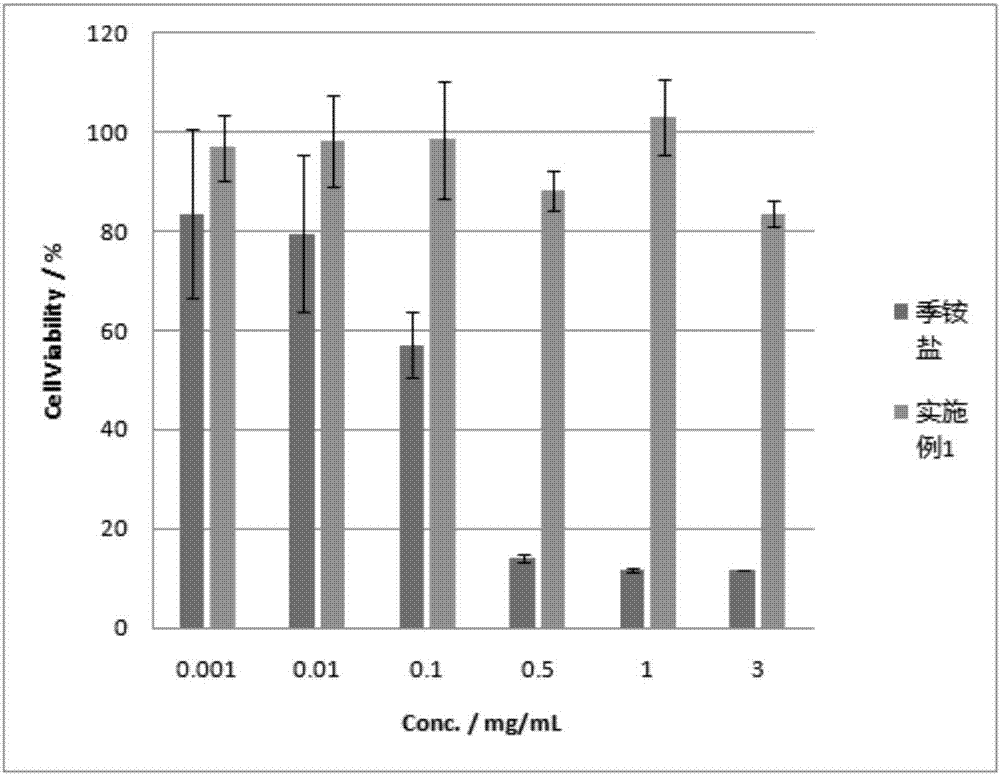
Novel water soluble natural polysaccharide antibacterial material and preparation method thereof
A technology of natural polysaccharides and antibacterial materials, applied in botany equipment and methods, chemicals for biological control, biocides, etc., can solve the problem that the antibacterial performance has not been significantly improved, so as to avoid the decline of antibacterial effect and operate Simple, less cytotoxic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Add 0.5 gram of chitosan to 100 milliliters of dilute hydrochloric acid with a concentration of 0.1 mol / L, and mechanically stir for half an hour under the condition of an oil bath at 60° C., so that the chitosan is completely dissolved, thereby obtaining a chitosan concentration of 0.005 g / L. mL of a homogeneous solution; the oil bath was heated to 110°C, and 1.3 grams of dicyandiamide was added to the chitosan solution at one time, and the molar ratio of dicyandiamide to chitosan was 5:1, and kept stirring at a constant temperature for 6 hours; The reaction solution was cooled to room temperature, and then lysine, N-hydroxysuccinimide (NHS) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide activated at room temperature for 2 hours were Mixed solution of hydrochloride (EDC·HCl) (the solvent is a buffer solution of 2-(N-morpholino)ethanesulfonic acid (MES) with a concentration of 30mmol / L, the pH value is about 5.0) 20mL is added to the above reaction solution In the pr...
Embodiment 2
[0056] Add 1.0 gram of chitosan into 100 milliliters of dilute hydrochloric acid with a concentration of 0.1 mol / L, and mechanically stir for one hour under an oil bath condition of 60° C., so that the chitosan dissolves completely, thereby obtaining a chitosan concentration of 0.01 g / L. mL of a homogeneous solution; the oil bath was heated to 105°C, and 1.05 grams of cyanamide was added once to the chitosan solution system, the molar ratio of cyanamide to chitosan was 4:1, and kept stirring at a constant temperature for 6 hours; then oil The reaction solution in the bath was cooled to room temperature, and the leucine, N-hydroxysuccinimide (NHS) and 1-(3-dimethylaminopropyl)-3-ethane activated for 3 hours were Add 20ml of a mixed solution of carbodiimide hydrochloride (EDC·HCl) (the buffer solution of 2-(N-morpholino)ethanesulfonic acid (MES) with a solvent of 30mmol / L) into the above reaction solution, Continue to stir and react at room temperature for 10 hours, wherein the ...
Embodiment 3
[0058] Add 2.0 grams of chitosan to 100 milliliters of dilute hydrochloric acid with a concentration of 0.15 mol / L, and mechanically stir for one hour under an oil bath condition of 60° C., so that the chitosan dissolves completely, thereby obtaining a chitosan concentration of 0.02 g / L. mL of uniform solution; heat the oil bath to 100°C, add 2.08 grams of dicyandiamide to the chitosan oil bath system at one time, the molar ratio of dicyandiamide to chitosan is 2:1, under the condition of 100°C oil bath Stir at constant temperature for 12 hours; then the reaction solution in the oil bath was cooled to room temperature, and the isoleucine, N-hydroxysuccinimide (NHS) and 1-(3-dimethyl Aminopropyl)-3-ethylcarbodiimide hydrochloride (EDC·HCl) mixed solution (the solvent is the buffer of 2-(N-morpholino)ethanesulfonic acid (MES) with a concentration of 30mmol / L Solution, pH value is about 5.0) 20ml is added to the above-mentioned reaction solution, and the stirring reaction is cont...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com