Preparation method and purpose of oxidation-reduction response chitosan-lipidosome
A chitosan and liposome technology, which can be used in liposome delivery, other methods of inserting foreign genetic materials, and medical preparations with inactive ingredients, etc. Application prospect, high biocompatibility, effect of improving biocompatibility and blood stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
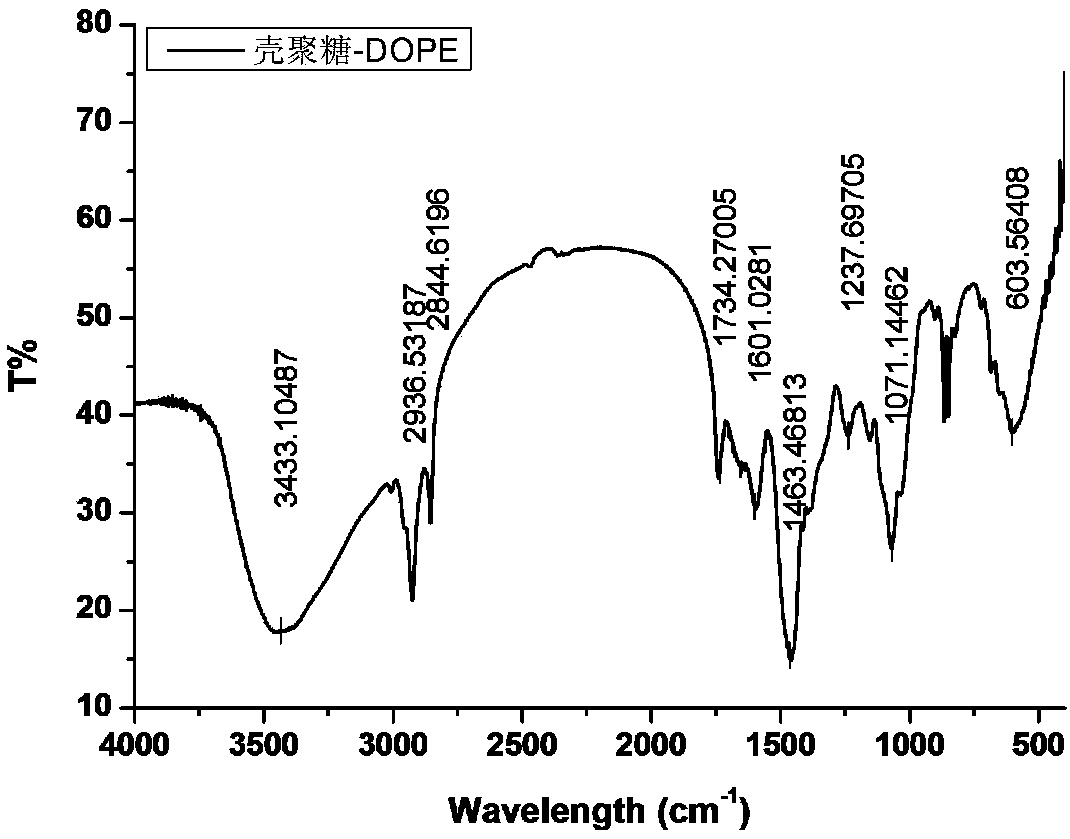
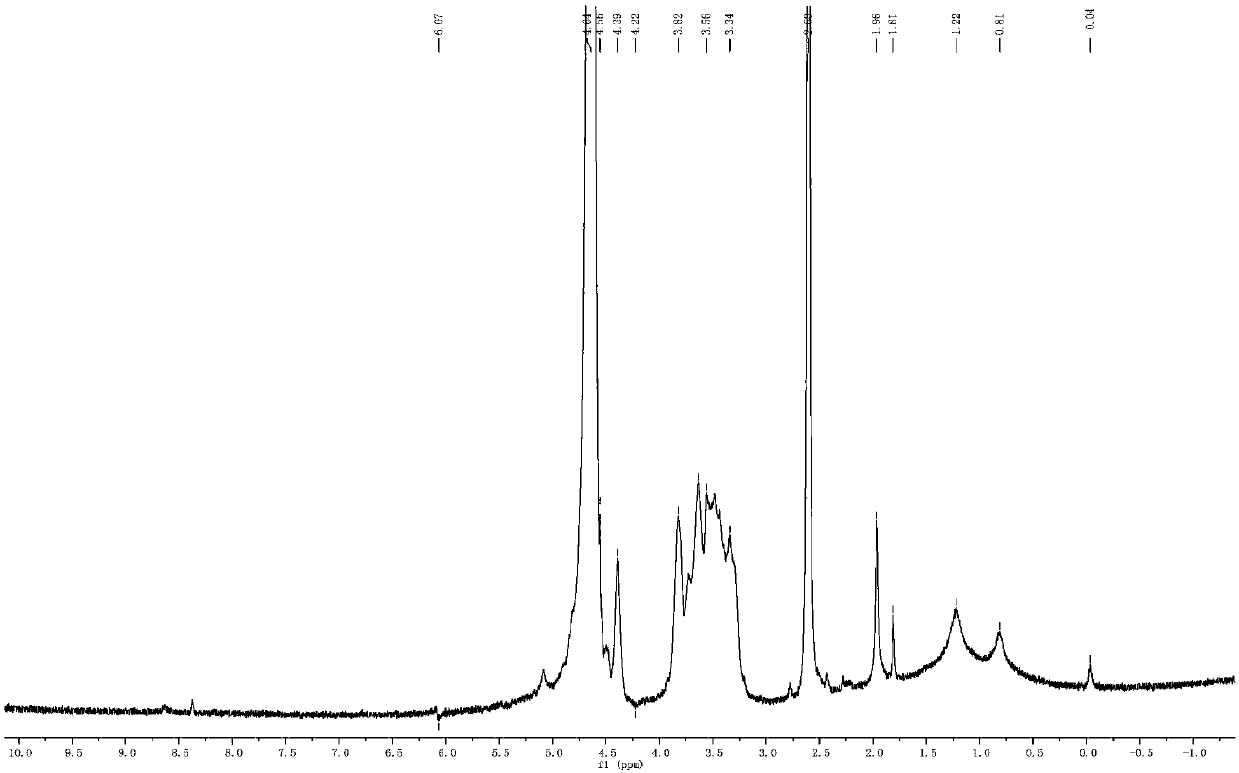
[0034]Dissolve 0.5 g of chitosan (CSO) with a molecular weight of 5 kDa in 100 mL of water, and sonicate for 30 min to fully dissolve it. Under the stirring state, add the chitosan aqueous solution dropwise to the DMSO solution of dithiobisuccinimidyl propionate, and after reacting at 30°C for 2 hours, continue to drop 0.5 g of 1,2 -Ethanol solution of dioleoylphosphatidylethanolamine, reacted at 30°C for 2h, the reaction solution was rotary evaporated, dialyzed and freeze-dried to prepare redox responsive 1,2-dioleoylphosphatidylethanolamine chitosan.
[0035] Prepare a 1mg / mL redox response 1,2-dioleoylphosphatidylethanolamine chitosan aqueous solution, take 100uL, mix it with 1mL of DOTAP cationic liposomes containing SPIO by ultrasound, then let it stand for 1h, and then insert it into the In the way of assembly, liposomes are modified to obtain liposome drug carriers with redox-responsive chitosan brushes on the surface.
Embodiment 2
[0037] Dissolve 0.5 g of chitosan (CSO) with a molecular weight of 5 kDa in 100 mL of water, and sonicate for 30 min to fully dissolve it. Under the stirring state, add the chitosan aqueous solution dropwise to the DMSO solution of dithiobisuccinimidyl propionate, and after reacting at 30°C for 2 hours, continue to drop 0.5 g of 1,2 - An ethanol solution of distearoylphosphatidylethanolamine, reacted at 30° C. for 2 hours, and the reaction solution was rotary evaporated, dialyzed and freeze-dried to prepare redox-responsive 1,2-distearoylphosphatidylethanolamine chitosan.
[0038] Prepare a 1mg / mL redox response 1,2-distearoylphosphatidylethanolamine chitosan aqueous solution, take 100uL, mix it with 1mL of DOTAP cationic liposome containing SPIO by ultrasound, then let it stand for 1h, and then insert In the way of self-assembly, liposomes are modified to obtain liposome drug carriers with redox-responsive chitosan brushes on the surface.
Embodiment 3
[0040] Dissolve 0.5 g of chitosan (CSO) with a molecular weight of 5 kDa in 100 mL of water, and sonicate for 30 min to fully dissolve it. Under the stirring state, add the chitosan aqueous solution dropwise to the DMSO solution of dithiobisuccinimidyl propionate, and after reacting at 30°C for 2 hours, continue to drop 0.5 g of 1,2 -Ethanol solution of dilauroylphosphatidylethanolamine, reacted at 30°C for 2h, the reaction solution was rotary evaporated, dialyzed and freeze-dried to prepare redox responsive 1,2-dilauroylphosphatidylethanolamine chitosan.
[0041] Prepare a 1mg / mL redox response 1,2-dilauroylphosphatidylethanolamine chitosan aqueous solution, take 100uL, mix it with 1mL of DOTAP cationic liposomes containing SPIO by ultrasound, then let it stand for 1h, and then insert it into the In the way of assembly, liposomes are modified to obtain liposome drug carriers with redox-responsive chitosan brushes on the surface.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com