Preparation method for copolymerize-modified graphite-phase carbon nitride visible light catalyst
A graphitic carbon nitride and copolymerization technology is applied in the directions of organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., to improve the performance of photo-splitting water for hydrogen production and the process is simple , the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] Preparation of graphitic carbon nitride. The invention uses urea as a raw material, and firstly calcines the urea in a muffle furnace to prepare graphite phase carbon nitride. The calcination temperature of urea is 500-550 degrees Celsius. The calcination time is 1-2 hours. Due to the reaction kinetics at this temperature, the polymerization is incomplete, and pure crystalline graphite phase carbon nitride cannot be obtained, and some amino defects (amino groups) will remain in the carbon nitride sheet structure.
[0026] The present invention uses graphite phase silicon nitride and small aromatic molecules as raw materials, mixes them uniformly, raises the temperature (the heating rate can be 5°C / min) to the boiling point temperature of the small aromatic molecules, and makes the small aromatic molecules The aldehyde group in the graphite phase undergoes a Schiff base reaction with the amino group on the edge of carbon nitride in the graphite phase, and is connected ...
Embodiment 1
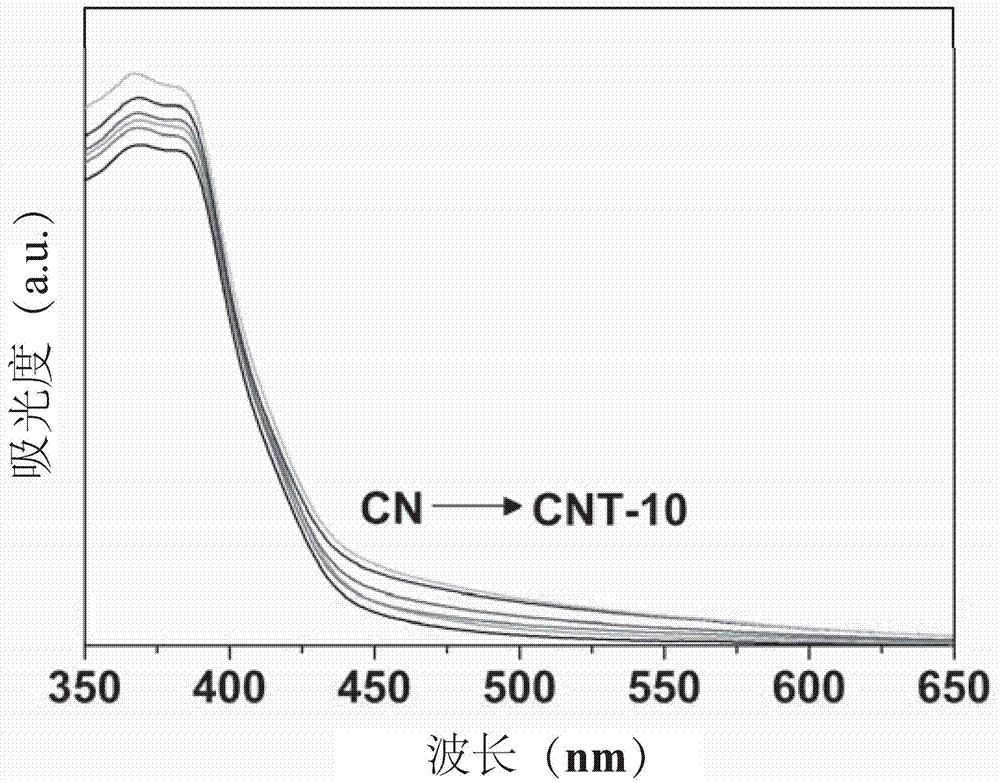
[0033] Weigh 20g urea into Al 2 o 3 Cover the crucible, place it in a muffle furnace for calcination, heat up to 550°C at a heating rate of 5°C / min, keep warm for 2h, cool naturally to room temperature, and grind to obtain graphitic carbon nitride (CN). Weigh 1g of the graphite-phase carbon nitride (CN) obtained above, and mix them with 1mg, 5mg, 10mg, 50mg, 0.1g of terephthalaldehyde in the solid phase, and then put in Al 2 o 3 Put a cover in the crucible, place it in a muffle furnace for calcination, heat up at a rate of 5°C / min, heat to 250°C, keep it warm for 5h, cool naturally to room temperature, and grind to obtain copolymerized modified graphite phase carbon nitride (CNT-x ), wherein T represents terephthalaldehyde, and x represents the mass percentage of terephthalaldehyde and CN added, and the values are 0.1, 0.5, 1, 5, 10. The UV-vis absorption spectrum of the prepared CNT-x visible light catalyst is as attached figure 1 shown by figure 1 It can be seen that ...
Embodiment 2
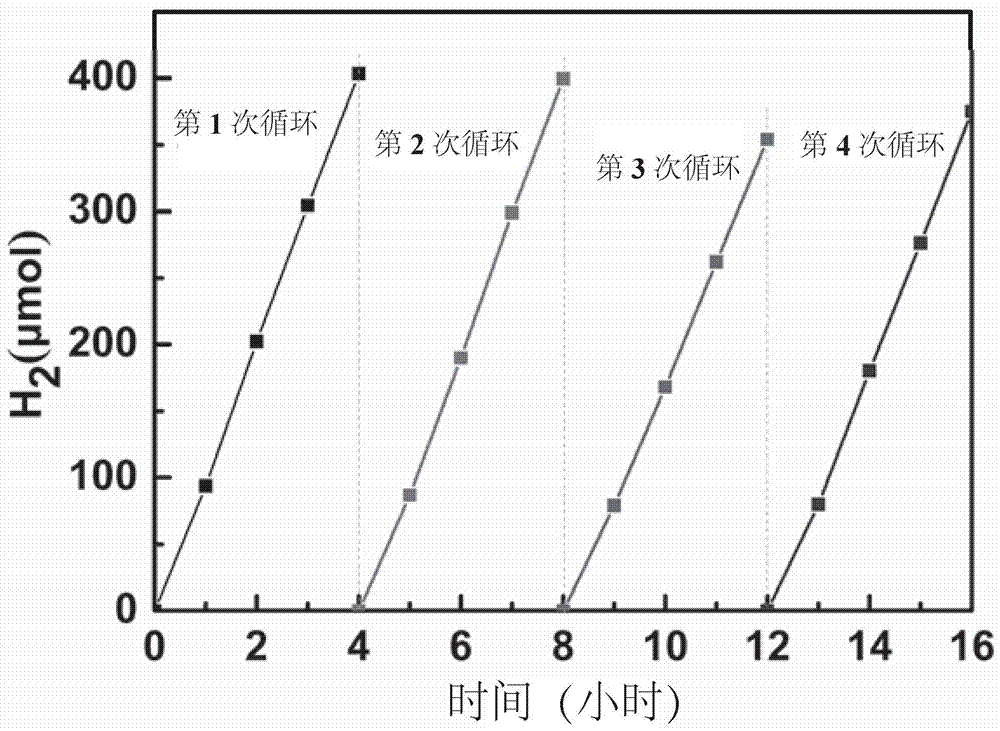
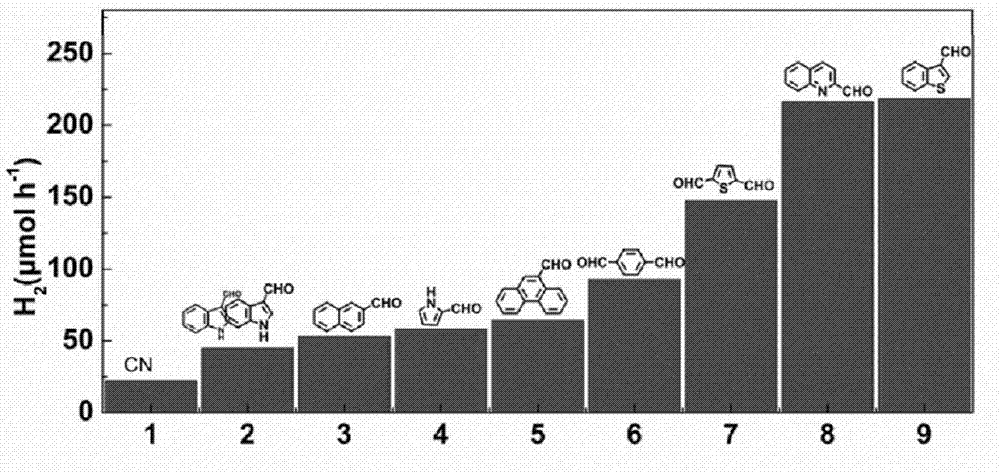
[0035] Weigh 20g of urea into Al 2 o 3 Cover the crucible, place it in a muffle furnace for calcination, heat up to 550°C at a heating rate of 5°C / min, keep warm for 2h, cool naturally to room temperature, and grind to obtain graphitic carbon nitride (CN). Weigh and take 1g of the graphite phase carbon nitride (CN) obtained above, and mix it with 1mg of different aldehyde-containing aromatic small molecules (indole-3-carbaldehyde, 2-naphthaldehyde, pyrrole-2-carbaldehyde, 9 - formaldehyde phenanthrene, terephthalaldehyde, 2,5-thiophene dicarbaldehyde, 2-quinoline formaldehyde, 3-formylbenzothiophene) solid phase mixing, put into Al 2 o 3 Cover the crucible, place it in a muffle furnace for calcination, heat up at a rate of 5°C / min, heat to the boiling point of small aromatic molecules, keep it warm for 5 hours, cool naturally to room temperature, and grind to obtain graphite phase nitrogen modified with different aromatic rings carbonized. The photohydrogen production diag...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com