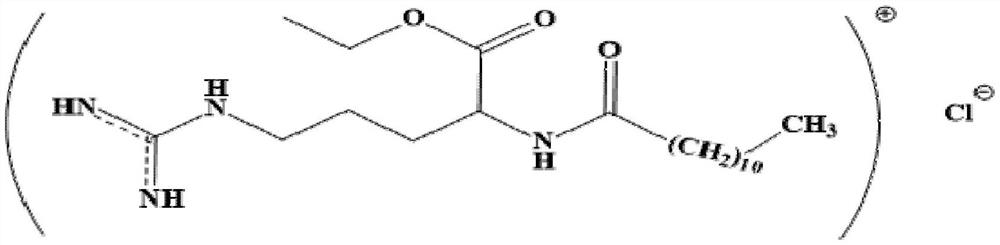
l-Lauramide arginine hydrochloride ethanol ester artificial antigen, preparation method of specific antibody and use thereof
A technology of lauramide and arginine is applied in the field of immunochemical analysis to achieve the effects of avoiding organic solvents, facilitating on-site monitoring and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1. Synthesis of artificial antigens
[0031] Based on lauroyl arginine (LAS) is the main component in the molecular structure of LAE, and can fully expose the structural features and contain carboxyl groups that can be coupled with proteins, it is used as a hapten.
[0032] The structure of a hapten is as follows:
[0033]
[0034] 1.1 Synthesis and purification of immunizing antigens
[0035] The carbodiimide method was used for the synthesis of immune antigens: 71.3 mg of lauroyl arginine LAS (about 0.2 mmol) was dissolved in 1 mL of N,N-dimethylformamide DMF, and 81.6 mg of N-hydroxysuccinic acid was added. Imide NHS (about 0.6 mmol), stirred at room temperature for 15 min, and then added 72.9 mg of dicyclohexylcarbodiimide DCC (0.3 mmol), stirred at room temperature overnight, centrifuged, and slowly added 0.5 mL of the supernatant to the mixture. In 10 mL of 10 mg / mL bovine serum albumin carbonate buffer solution, the reaction was magnetically stirred for 5 ho...
Embodiment 2
[0047] 2. Synthesis of artificial antigens
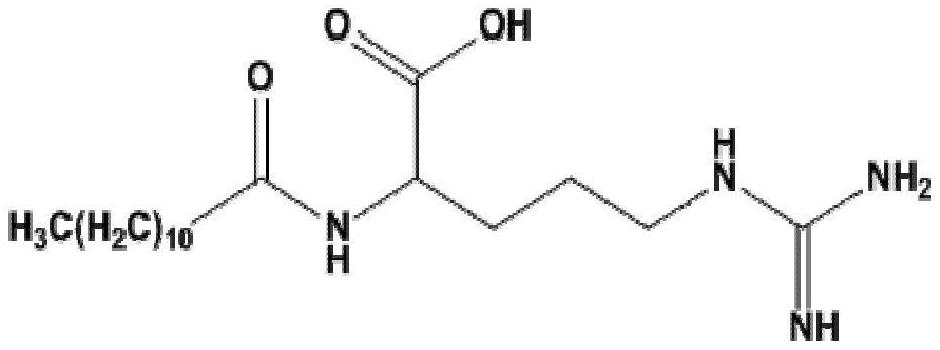
[0048] Based on lauroyl arginine (LAS) is the main component in the molecular structure of LAE, and can fully expose the structural features and contain carboxyl groups that can be coupled with proteins, it is used as a hapten.
[0049] The structure of a hapten is as follows:
[0050]
[0051] 2.1 Synthesis and purification of immunizing antigens
[0052] The carbodiimide method was used for the synthesis of immune antigens: 107.0 mg of lauroyl arginine LAS (about 0.3 mmol) was dissolved in 2 mL of N,N-dimethylformamide DMF, and 122.4 mg of N-hydroxysuccinic acid was added. Imide NHS (0.9 mmol), stirred at room temperature for 15 min, then added 109.4 mg of dicyclohexylcarbodiimide DCC (0.45 mmol), stirred at room temperature overnight, centrifuged, and slowly added 0.8 mL of supernatant to 15 mL of 15 mg / mL of bovine serum albumin carbonate buffer solution, magnetic stirring for 5.5 hours. The solution after the reaction wa...
Embodiment 3
[0058] 3. Synthesis of artificial antigens
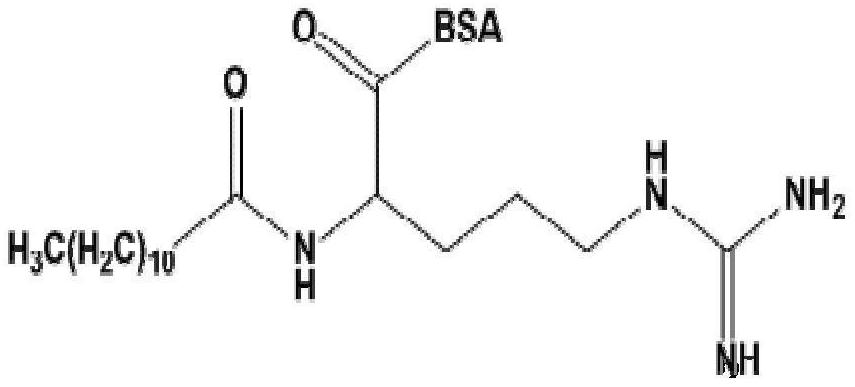
[0059] Based on lauroyl arginine (LAS) is the main component in the molecular structure of LAE, and can fully expose the structural features and contain carboxyl groups that can be coupled with proteins, it is used as a hapten.
[0060] The structure of a hapten is as follows:
[0061] 3.1 Synthesis and purification of immunizing antigens
[0062] The carbodiimide method was used for the synthesis of immune antigens: 142.6 mg of lauroyl arginine LAS (about 0.4 mmol) was dissolved in 1.5 mL of N,N-dimethylformamide DMF, and 163.2 mg of N-hydroxyl was added. Succinimide NHS (1.2 mmol) was stirred at room temperature for 15 min, and then 145.8 mg of dicyclohexylcarbodiimide DCC (0.6 mmol) was added. The reaction was stirred at room temperature overnight, centrifuged, and 1 mL of the supernatant was slowly added to 20 mL. 20mg / mL bovine serum albumin carbonate buffer solution, magnetic stirring for 6 hours. The solution after the r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com