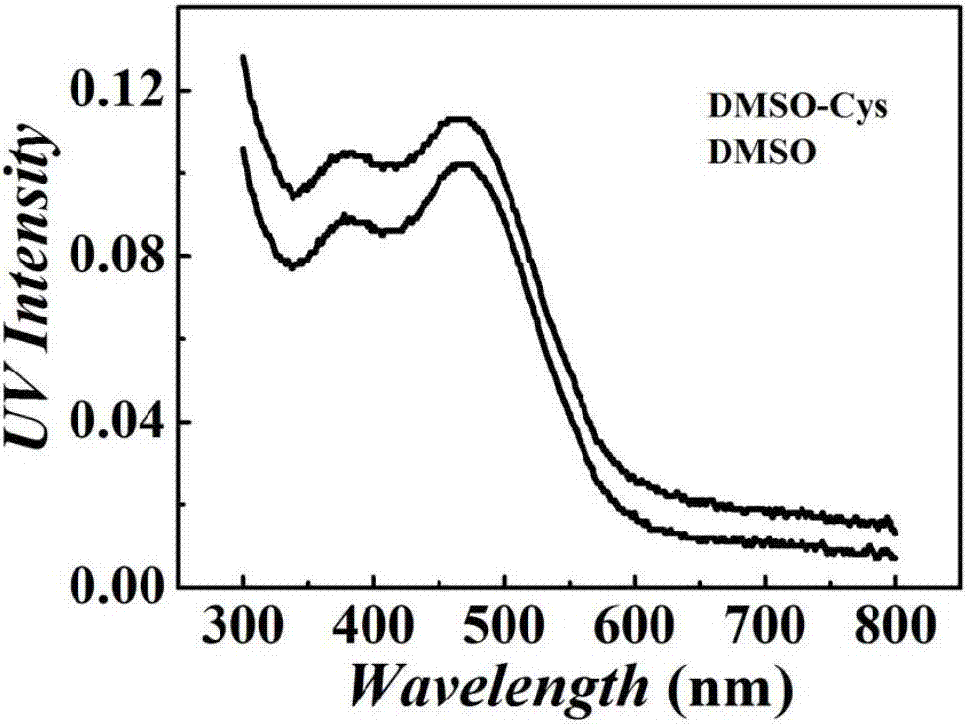
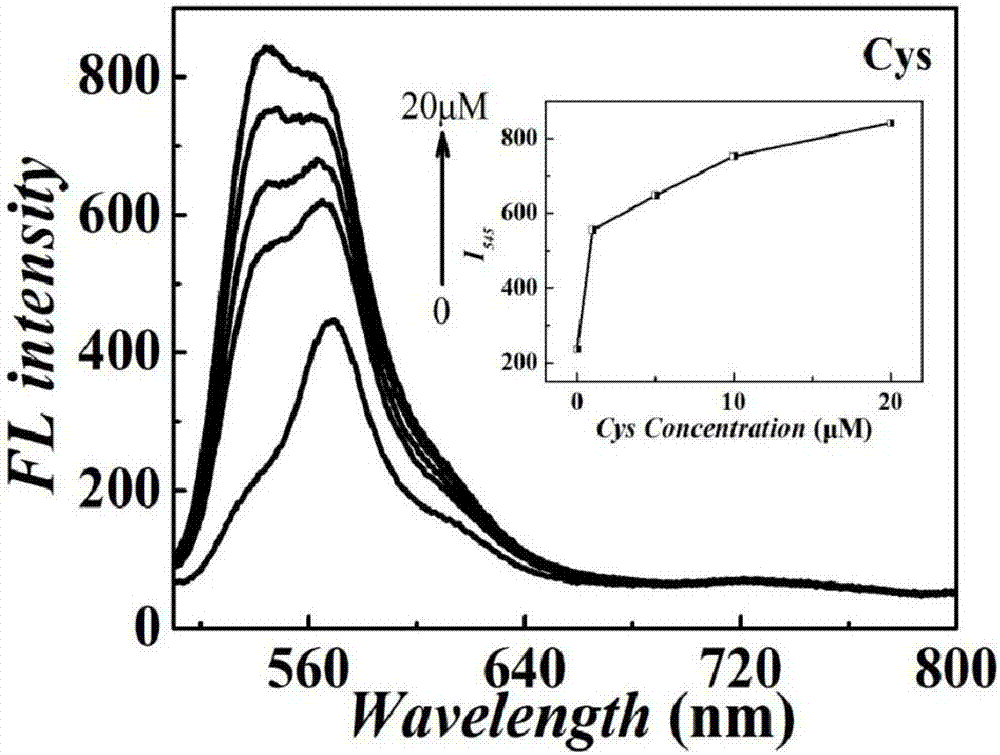
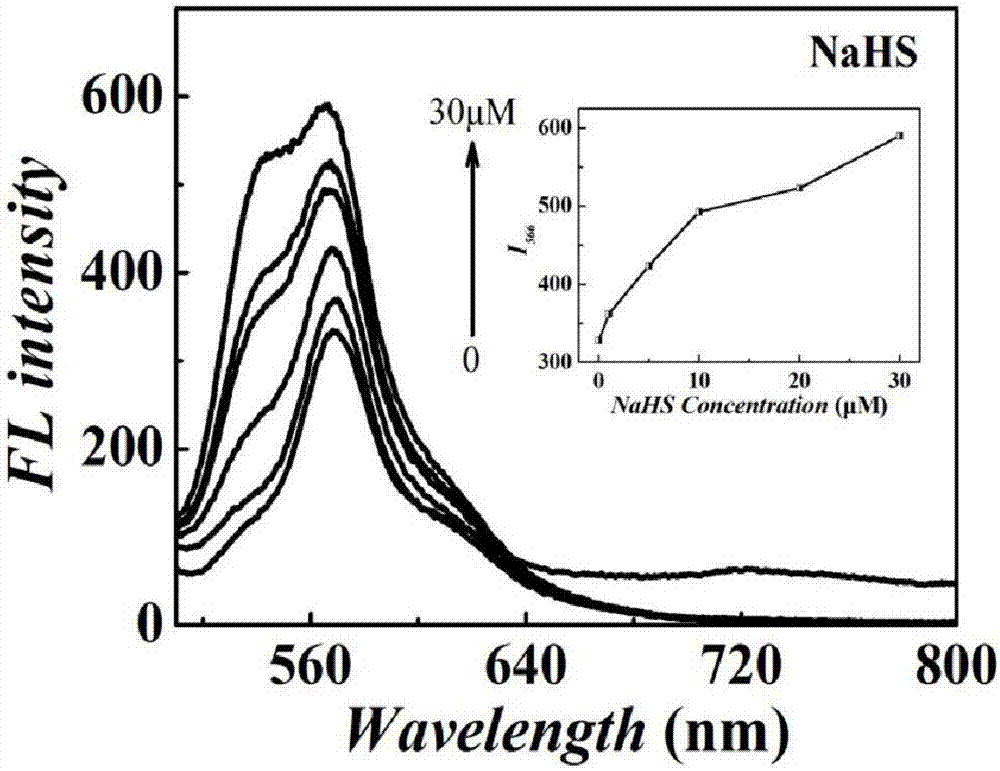
Tetraphenylethylene indole derivative, preparation method therefor and application of tetraphenylethylene indole derivative in cell imaging and thiol compound analysis
A technology of indole derivatives and tetraphenylethylene, which is applied in the field of bioluminescent probes, can solve the problems that restrict the application of fluorescence emission of organic light-emitting molecules, and achieve the effects of sensitive potential indication results and low biological toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The synthesis of tetraphenylethylene derivatives, concrete steps are as follows:
[0034] The first step: weigh 4,4-dimethoxydiphenyl ketone (7.3g, 0.03M), 4,4-dibromodiphenyl ketone (10.2g, 0.03M) and zinc powder (15.7g, 0.24M) was placed in a 500mL two-necked flask, and 200mL of anhydrous tetrahydrofuran was quickly added as a reaction solvent. The reaction bottle is well connected with the condensing device, and the whole reaction system is in a sealed state. Air was removed from the system at -78°C. Slowly inject 0.12 mol of titanium tetrachloride (13.16 mL) at low temperature (0°C). After returning to room temperature, the reaction system was transferred to an oil bath, heated to 80°C and stirred for 9 hours. After the reaction is completed, add an appropriate amount of saturated potassium carbonate solution to terminate the reaction, filter, extract the crude product with water and dichloromethane, spin the dichloromethane part to obtain the crude product, and ...
Embodiment 2
[0042] Cytotoxicity experiments, the steps are as follows:
[0043] 1) Add 100 μL / well (approximately 1×10 4 ), set at 37°C 5% CO 2 Cultured in a cell culture incubator for 24 hours.
[0044] 2) Add 1-500 μg / L tetraphenylethylene derivatives.
[0045] 3) Place the 96-well plate at 37°C in 5% CO 2 The cells were cultured in an incubator for 24 hours.
[0046] 4) Add 50 μL of 1×MTT to each well and incubate at 37° C. for 4 hours to reduce MTT to formazan.
[0047] 5) Aspirate the supernatant, add 150 μL DMSO to each well to make formazan, and shake well with a plate shaker.
[0048] 6) A microplate reader detects the optical density of each well at a wavelength of 490nm.
Embodiment 3
[0050] Cell imaging experiments, the steps are as follows:
[0051] 1) Human cervical cancer cells were cultured for 24 hours in a 35mm surface dish with a cover glass.
[0052] 2), configuration 10 -3 μmol / L tetraphenylethylene derivatives were co-cultured with the cultured cells for 30min.
[0053] 3) Carry out confocal imaging under 488nm excitation light.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com